Abstract
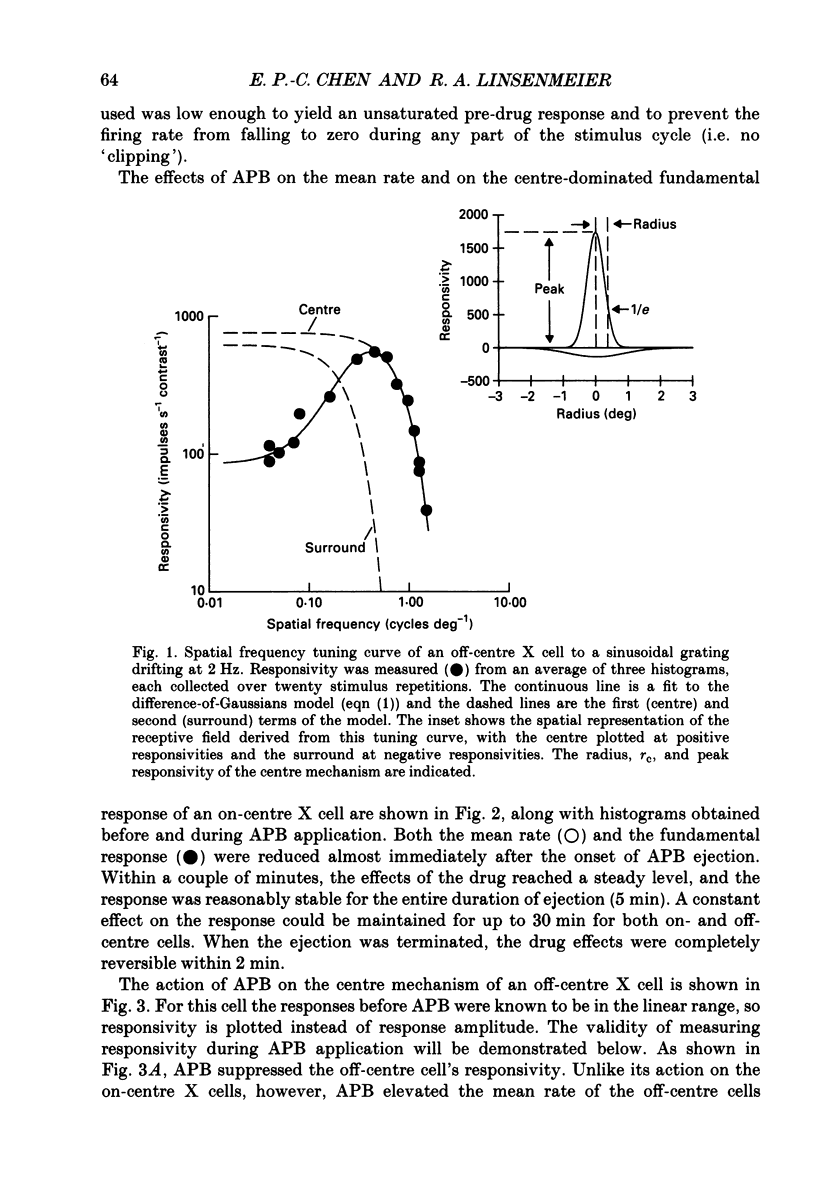
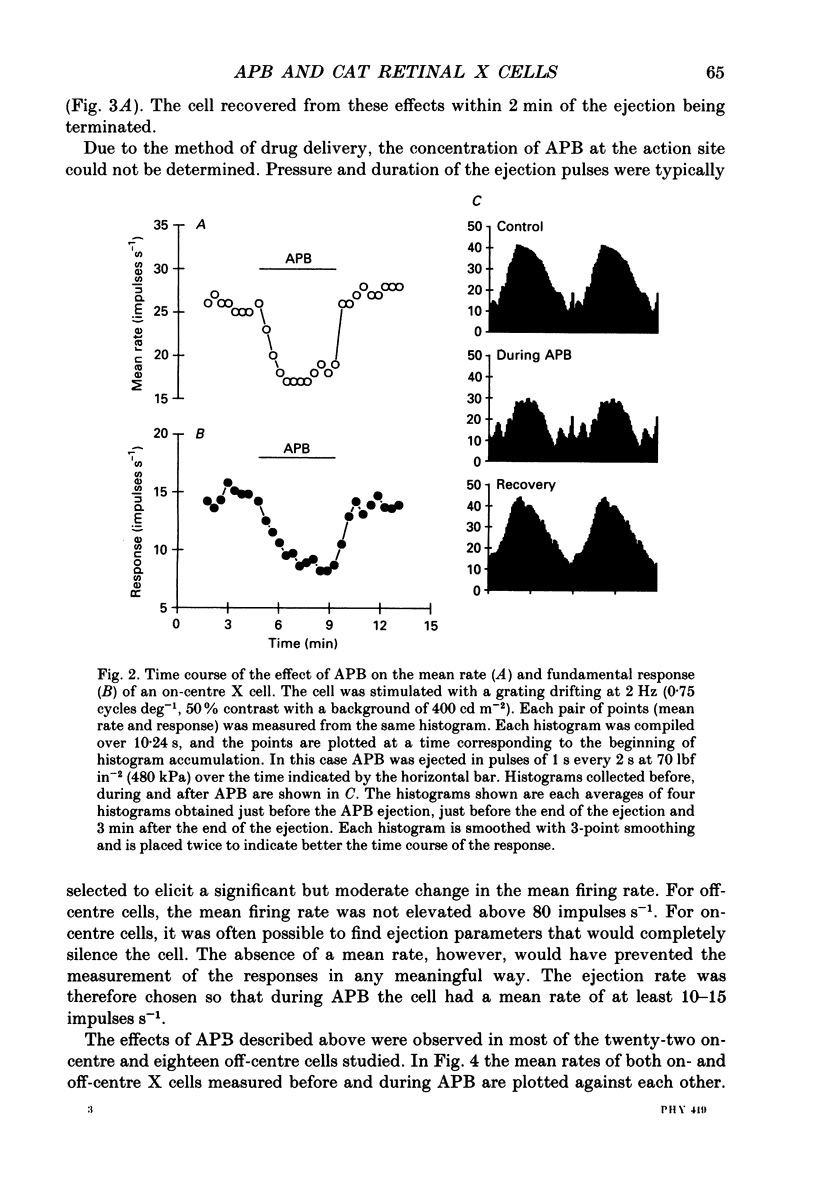
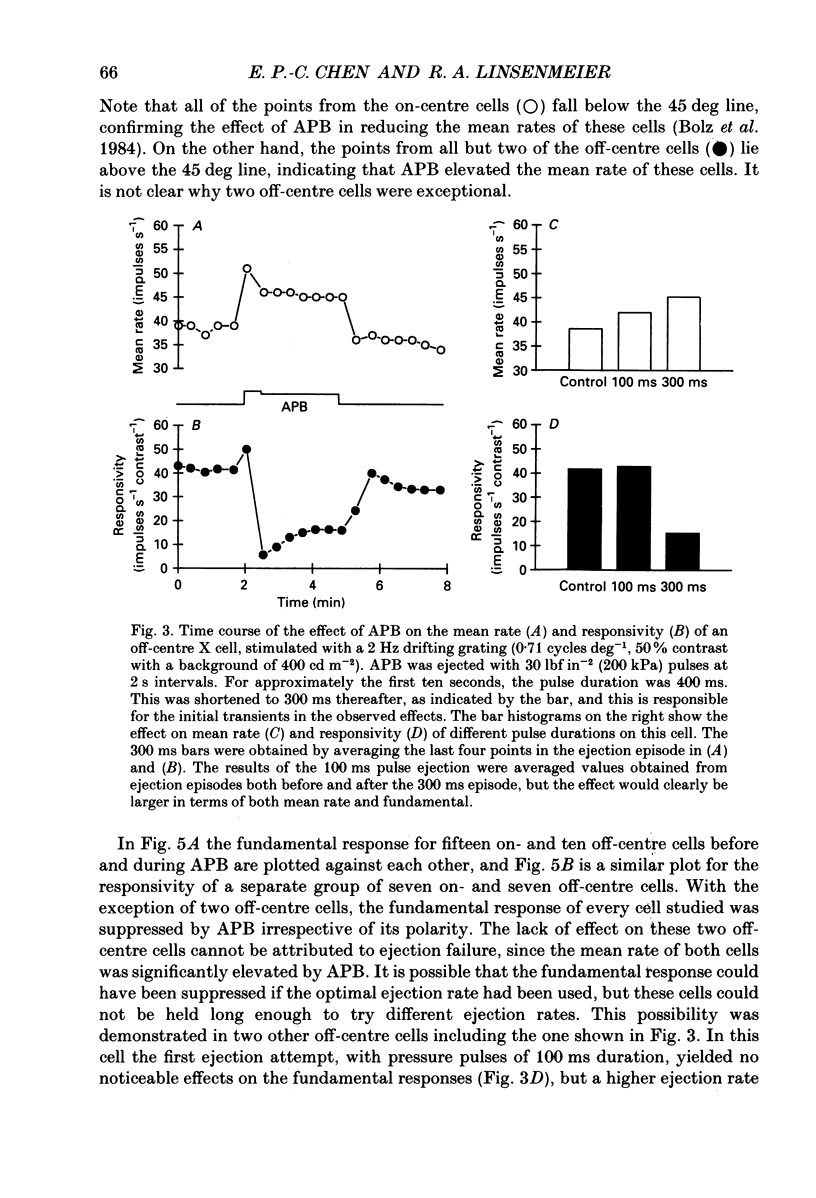
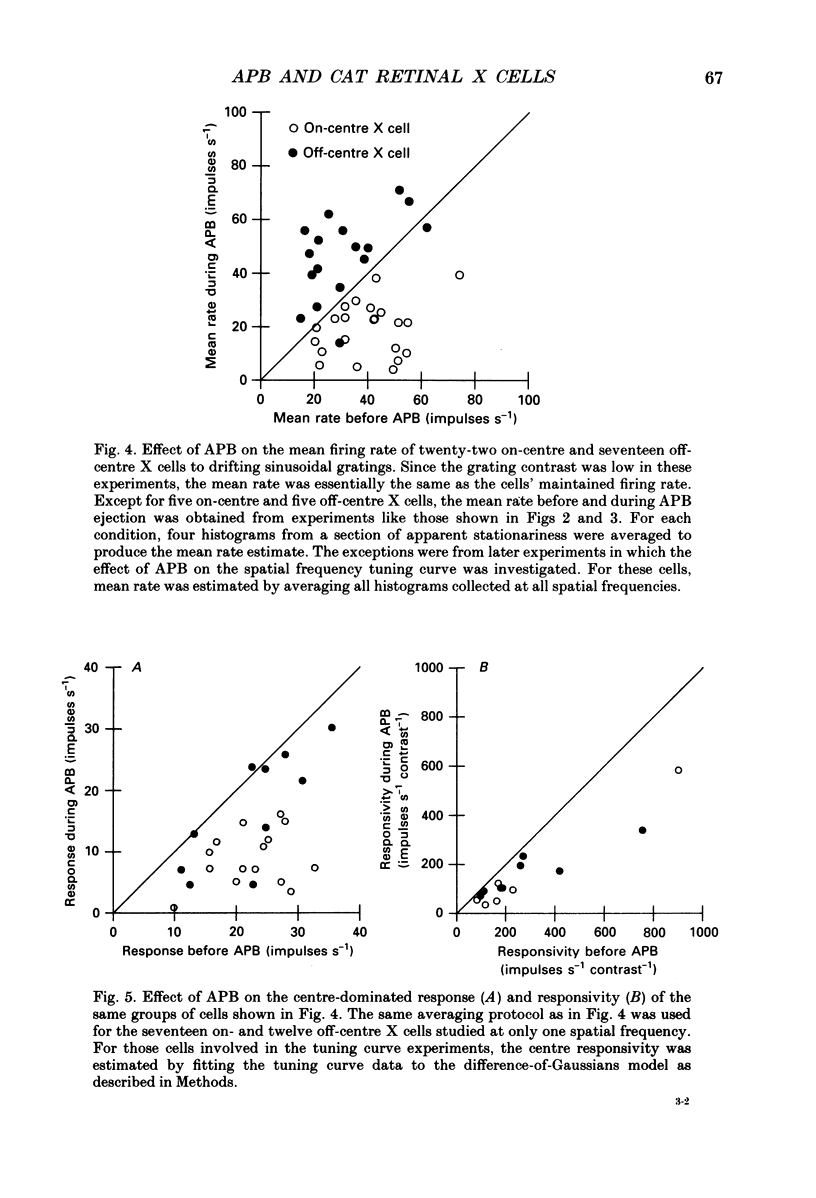
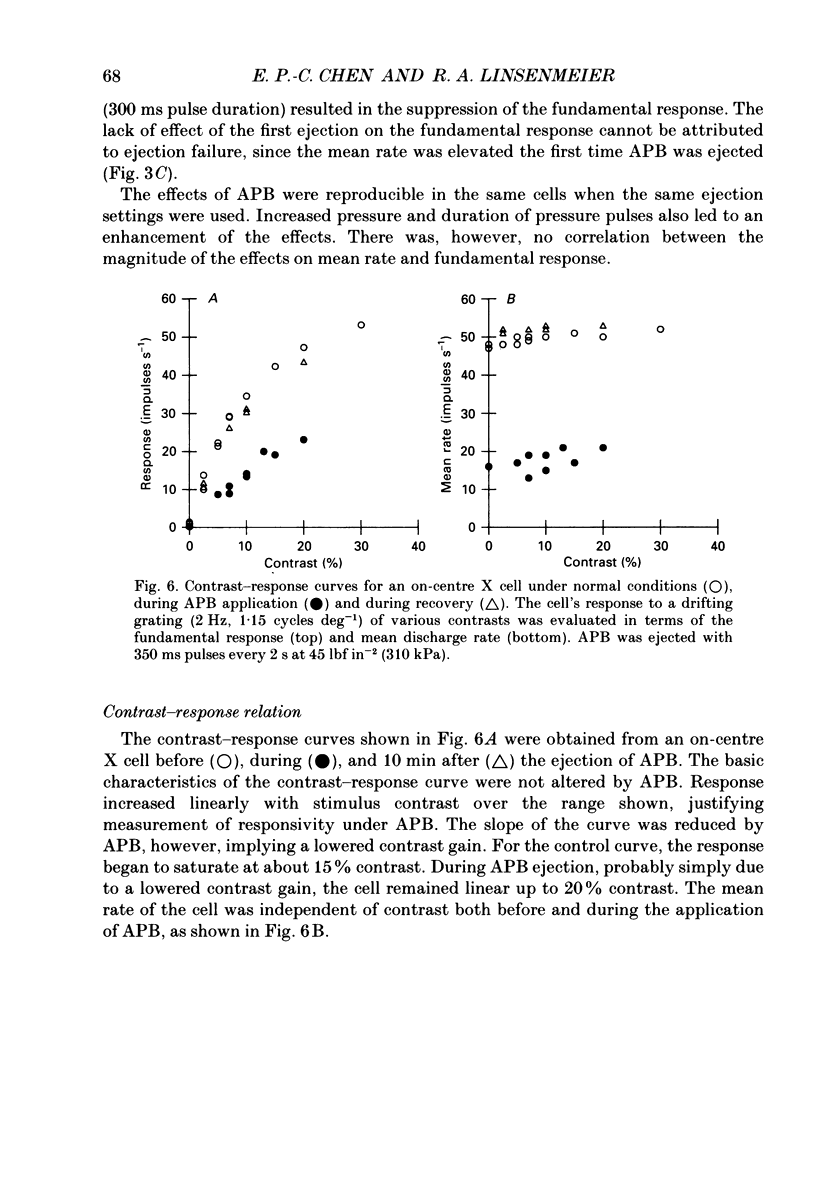
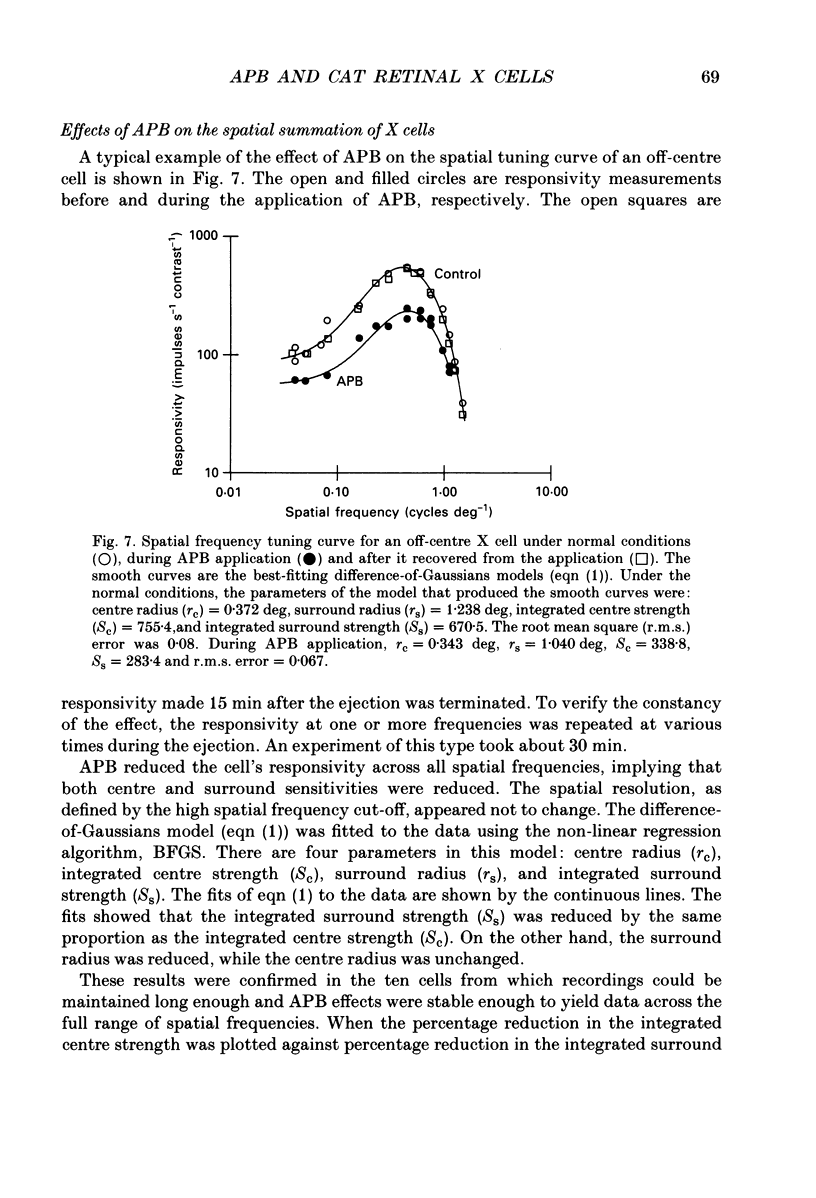
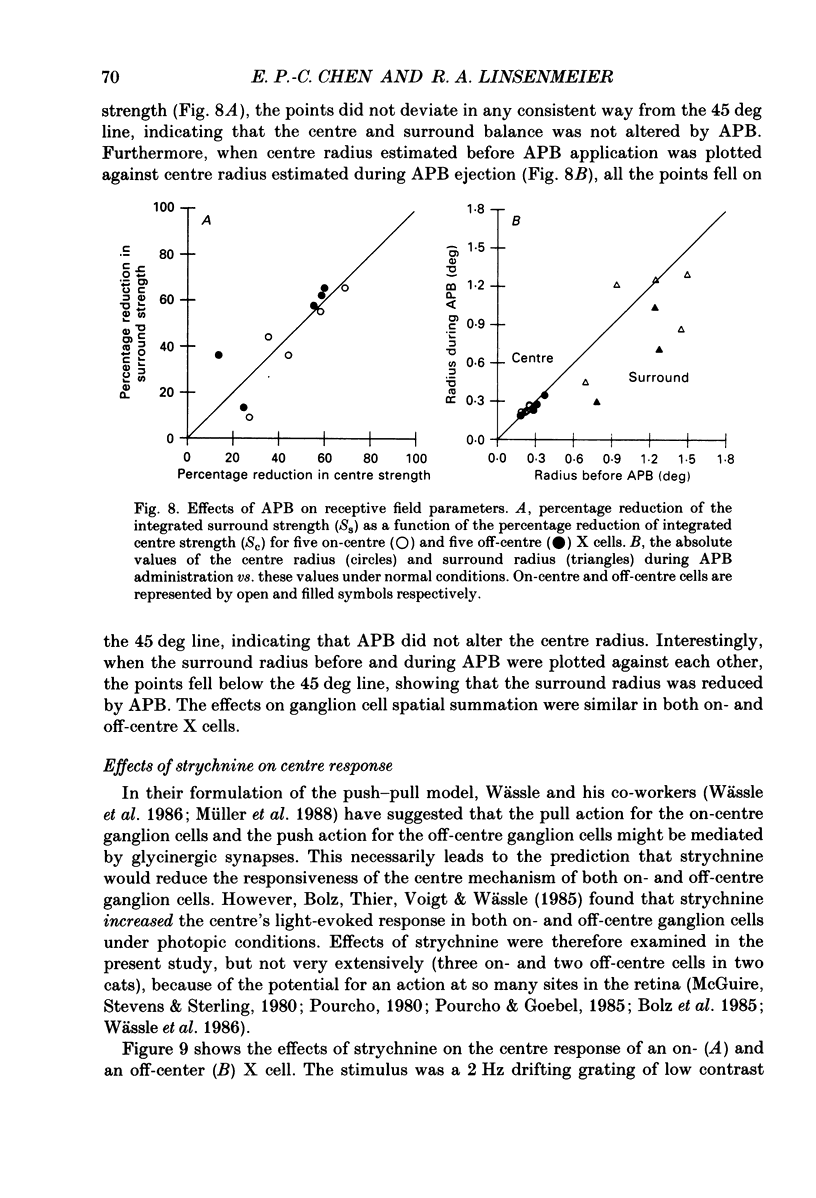
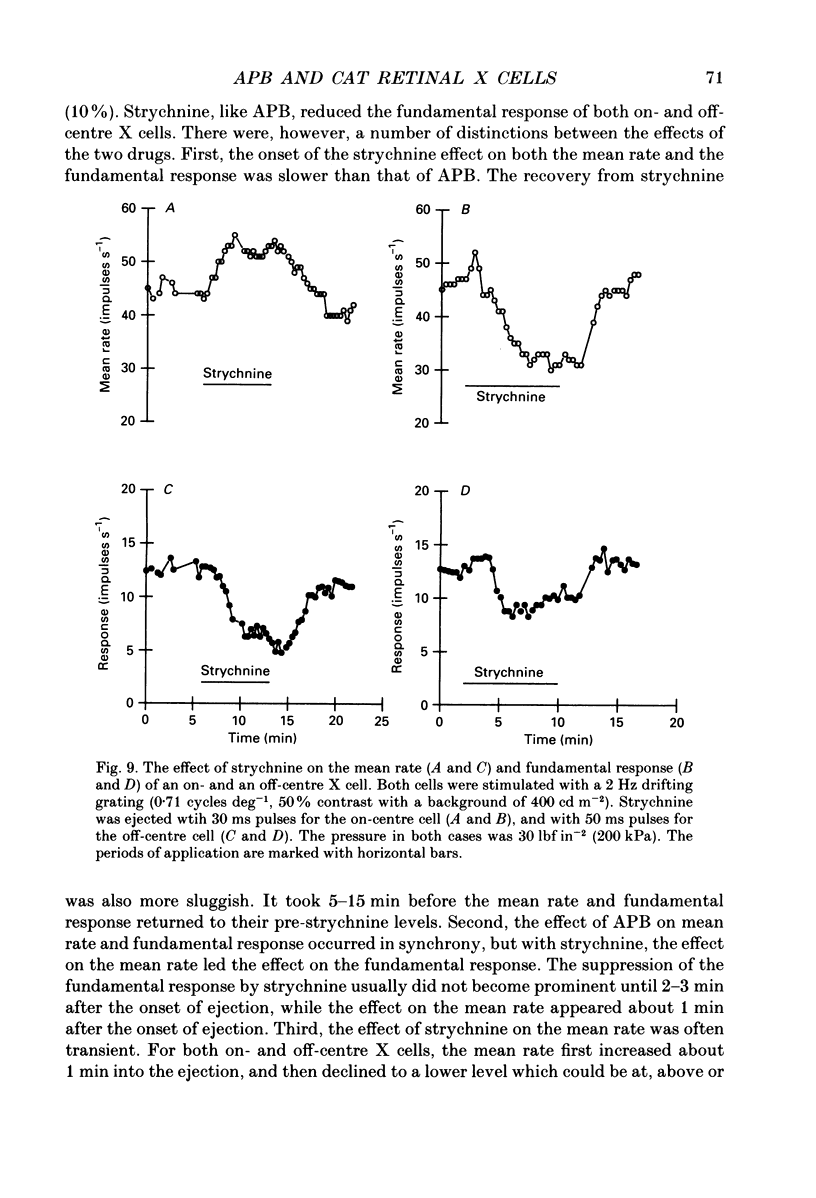
1. We studied the effect of locally applied 2-amino-4-phosphonobutyric acid (APB) on the response of X-type cat retinal ganglion cells to stimulation by sinusoidal gratings of photopic mean luminance. 2. Application of APB produced a decrease in the mean firing rate of on-centre X cells, but an increase in the mean firing rate of off-centre X cells. 3. The response and responsivity of both on- and off-centre X cells to drifting sinusoidal gratings were suppressed by APB. Under control conditions, the response amplitude was linear with grating contrast for contrasts less than 10%. During APB application, the slope of the contrast response curve was reduced, and linearity was maintained for a larger range of contrast. 4. Assuming that APB acts selectively on the cone to depolarizing bipolar cell synapse, the suppression of the response of on- and off-centre X cells suggests a functional link between depolarizing bipolar cells and both types of X cell. 5. Because APB reduces the centre response of both on- and off-centre X cells, caution should be observed in interpreting the effect of APB on higher visual centres in terms of the blockage of 'on' signals leaving the retina. 6. By analysing the spatial frequency tuning curve before and during APB application in terms of the difference-of-Gaussians model of receptive field structure, it was found that the balance between the integrated strengths of the centre and surround was not modified by APB. APB, however, had a differential effect on the summating area of the centre and surround. While the centre radius was unaffected by APB, the surround radius was reduced. This suggests that the peak sensitivity of the surround mechanism was reduced less severely than the peak sensitivity of the centre mechanism. 7. Strychnine also suppressed the response of the centre mechanism of both on- and off-centre X cells to drifting gratings.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloomfield S. A., Dowling J. E. Roles of aspartate and glutamate in synaptic transmission in rabbit retina. I. Outer plexiform layer. J Neurophysiol. 1985 Mar;53(3):699–713. doi: 10.1152/jn.1985.53.3.699. [DOI] [PubMed] [Google Scholar]
- Bolz J., Thier P., Voigt T., Wässle H. Action and localization of glycine and taurine in the cat retina. J Physiol. 1985 May;362:395–413. doi: 10.1113/jphysiol.1985.sp015685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz J., Wässle H., Thier P. Pharmacological modulation of on and off ganglion cells in the cat retina. Neuroscience. 1984 Jul;12(3):875–885. doi: 10.1016/0306-4522(84)90176-3. [DOI] [PubMed] [Google Scholar]
- Chen E. P., Linsenmeier R. A. Centre components of cone-driven retinal ganglion cells: differential sensitivity to 2-amino-4-phosphonobutyric acid. J Physiol. 1989 Dec;419:77–93. doi: 10.1113/jphysiol.1989.sp017862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Hertz B. G., Lennie P. Convergence of rod and cone signals in the cat's retina. J Physiol. 1977 Jul;269(2):297–318. doi: 10.1113/jphysiol.1977.sp011903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Pinto L. H. Gallamine triethiodide (flaxedil) and cat retinal ganglion cell responses. J Physiol. 1970 Jul;208(3):677–689. doi: 10.1113/jphysiol.1970.sp009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G., Schweitzer-Tong D. E., Watson A. B. Spatio-temporal interactions in cat retinal ganglion cells showing linear spatial summation. J Physiol. 1983 Aug;341:279–307. doi: 10.1113/jphysiol.1983.sp014806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frishman L. J., Linsenmeier R. A. Effects of picrotoxin and strychnine on non-linear responses of Y-type cat retinal ganglion cells. J Physiol. 1982 Mar;324:347–363. doi: 10.1113/jphysiol.1982.sp014116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J Physiol. 1976 Nov;262(2):265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. C., Sherk H. Receptive field properties in the cat's lateral geniculate nucleus in the absence of on-center retinal input. J Neurosci. 1984 Feb;4(2):374–380. doi: 10.1523/JNEUROSCI.04-02-00374.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby A. W. The effect of strychnine, bicuculline, and picrotoxin on X and Y cells in the cat retina. J Gen Physiol. 1979 Jul;74(1):71–84. doi: 10.1085/jgp.74.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier R. A., Frishman L. J., Jakiela H. G., Enroth-Cugell C. Receptive field properties of x and y cells in the cat retina derived from contrast sensitivity measurements. Vision Res. 1982;22(9):1173–1183. doi: 10.1016/0042-6989(82)90082-7. [DOI] [PubMed] [Google Scholar]
- Massey S. C., Redburn D. A. Transmitter circuits in the vertebrate retina. Prog Neurobiol. 1987;28(1):55–96. doi: 10.1016/0301-0082(87)90005-0. [DOI] [PubMed] [Google Scholar]
- McGuire B. A., Stevens J. K., Sterling P. Microcircuitry of beta ganglion cells in cat retina. J Neurosci. 1986 Apr;6(4):907–918. doi: 10.1523/JNEUROSCI.06-04-00907.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F., Wässle H., Voigt T. Pharmacological modulation of the rod pathway in the cat retina. J Neurophysiol. 1988 Jun;59(6):1657–1672. doi: 10.1152/jn.1988.59.6.1657. [DOI] [PubMed] [Google Scholar]
- Nelson R., Kolb H. Synaptic patterns and response properties of bipolar and ganglion cells in the cat retina. Vision Res. 1983;23(10):1183–1195. doi: 10.1016/0042-6989(83)90032-9. [DOI] [PubMed] [Google Scholar]
- Pourcho R. G., Goebel D. J. A combined Golgi and autoradiographic study of (3H)glycine-accumulating amacrine cells in the cat retina. J Comp Neurol. 1985 Mar 22;233(4):473–480. doi: 10.1002/cne.902330406. [DOI] [PubMed] [Google Scholar]
- Saito H. The effects of strychnine and bicuculline on the responses of X- and Y-cells of the isolated eye-cut preparation of the cat. Brain Res. 1981 May 11;212(1):243–248. doi: 10.1016/0006-8993(81)90061-5. [DOI] [PubMed] [Google Scholar]
- Schiller P. H. Central connections of the retinal ON and OFF pathways. Nature. 1982 Jun 17;297(5867):580–583. doi: 10.1038/297580a0. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981 Jan 9;211(4478):182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. The role of excitatory amino acid transmitters in the mudpuppy retina: an analysis with kainic acid and N-methyl aspartate. J Neurosci. 1983 Aug;3(8):1701–1711. doi: 10.1523/JNEUROSCI.03-08-01701.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. H., Walker M. L., Johnson W. M. A new microelectrode positioner for intraretinal recording from the intact mammalian eye. Vision Res. 1968 Dec;8(12):1521–1523. doi: 10.1016/0042-6989(68)90126-0. [DOI] [PubMed] [Google Scholar]


