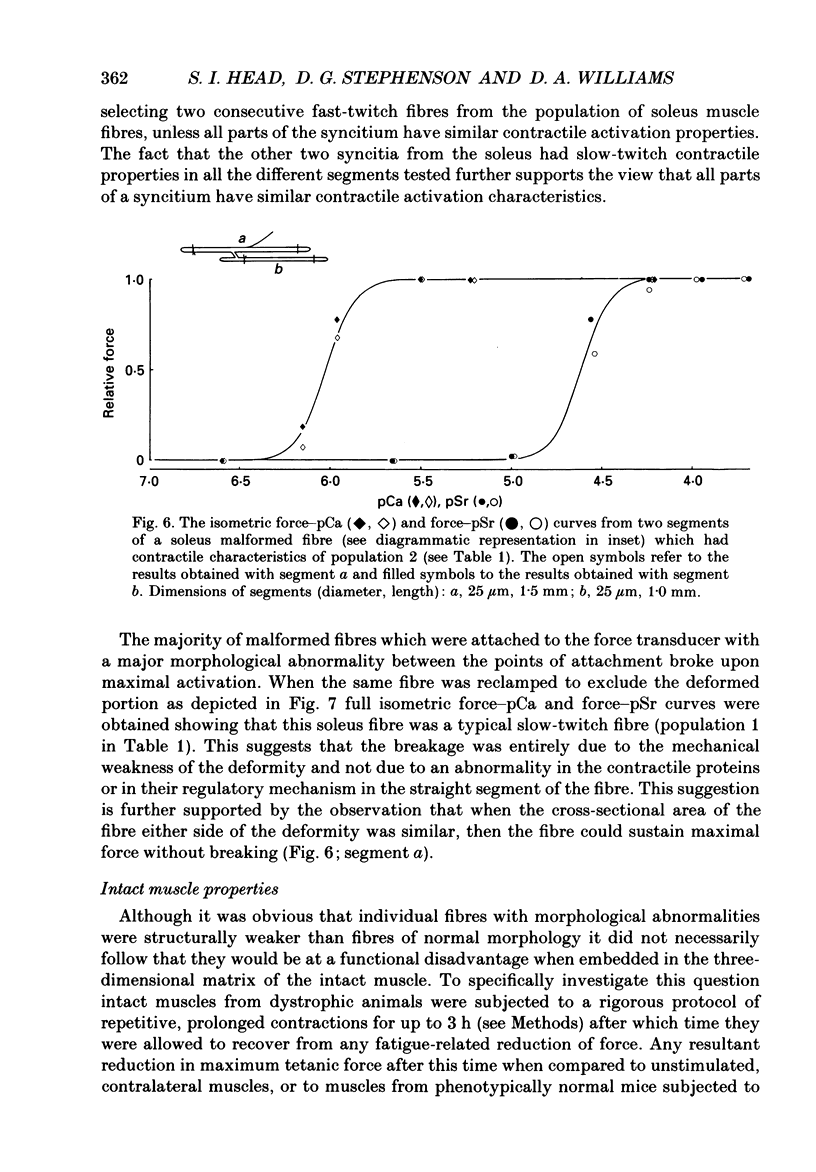
Abstract
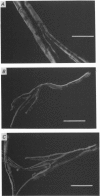
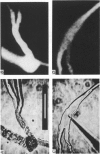
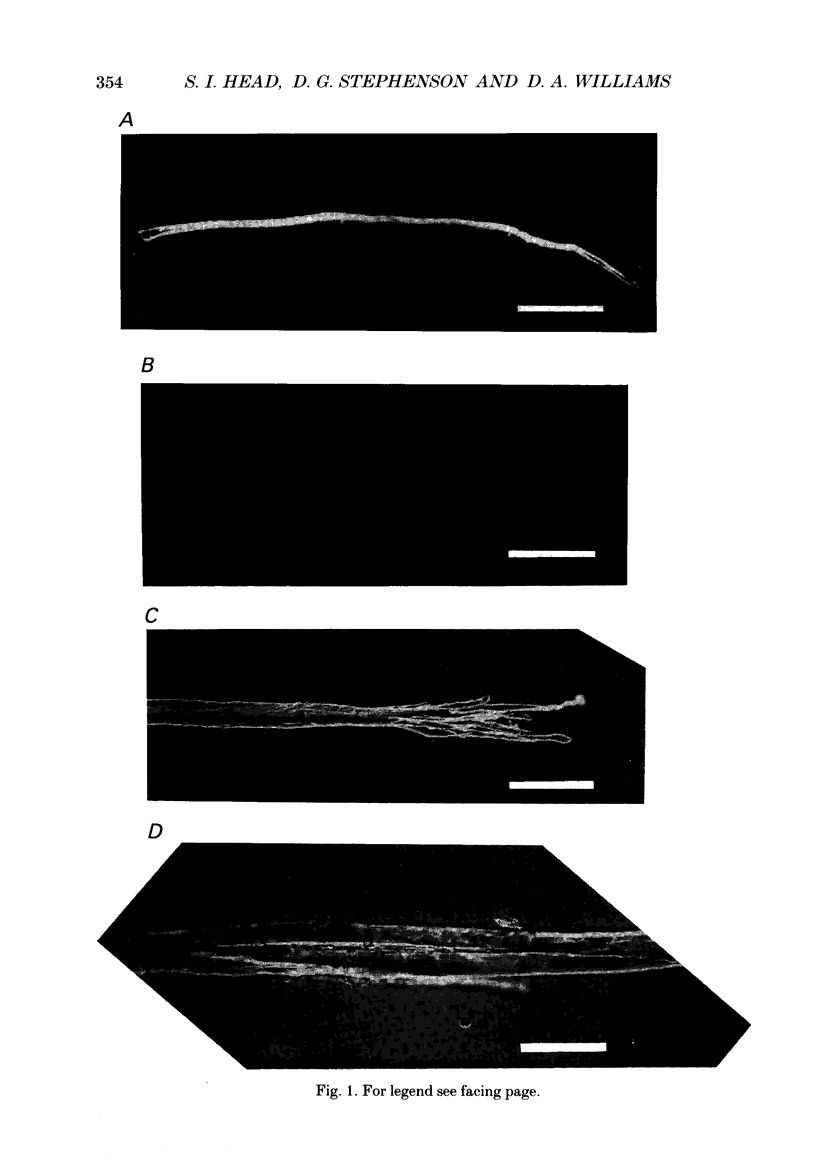
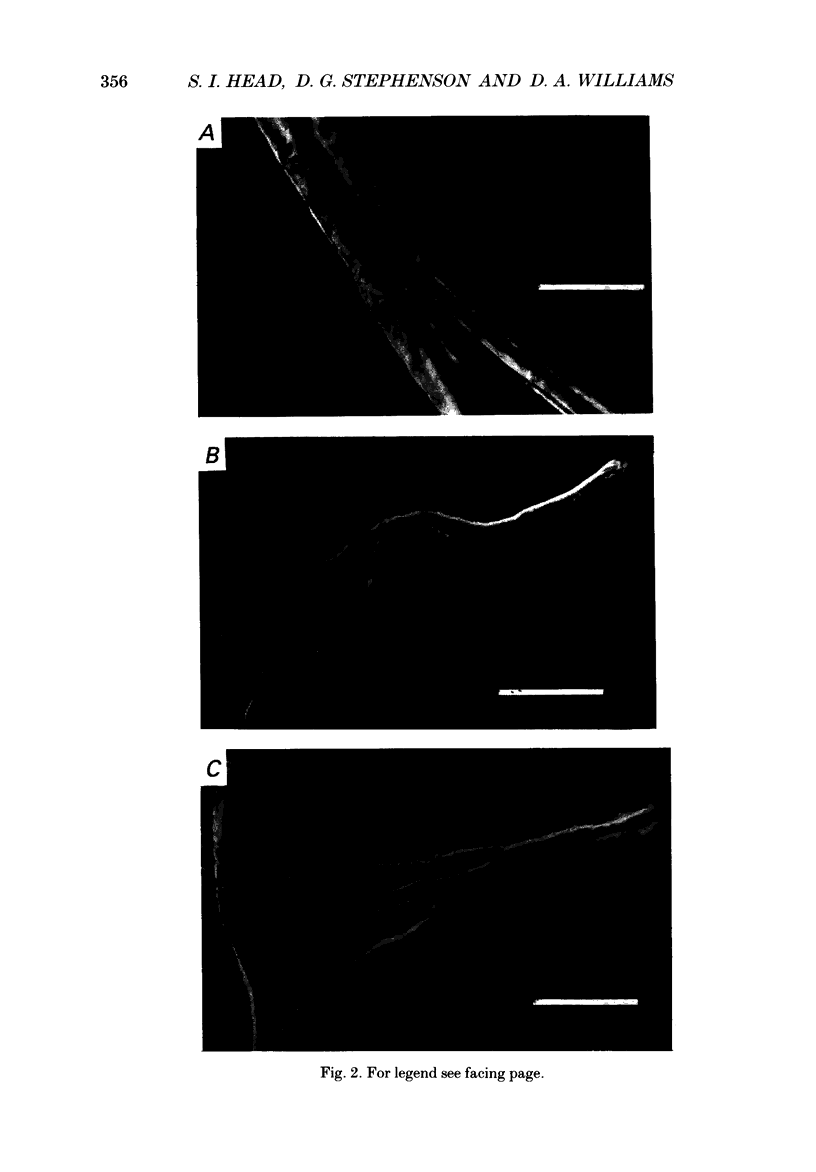
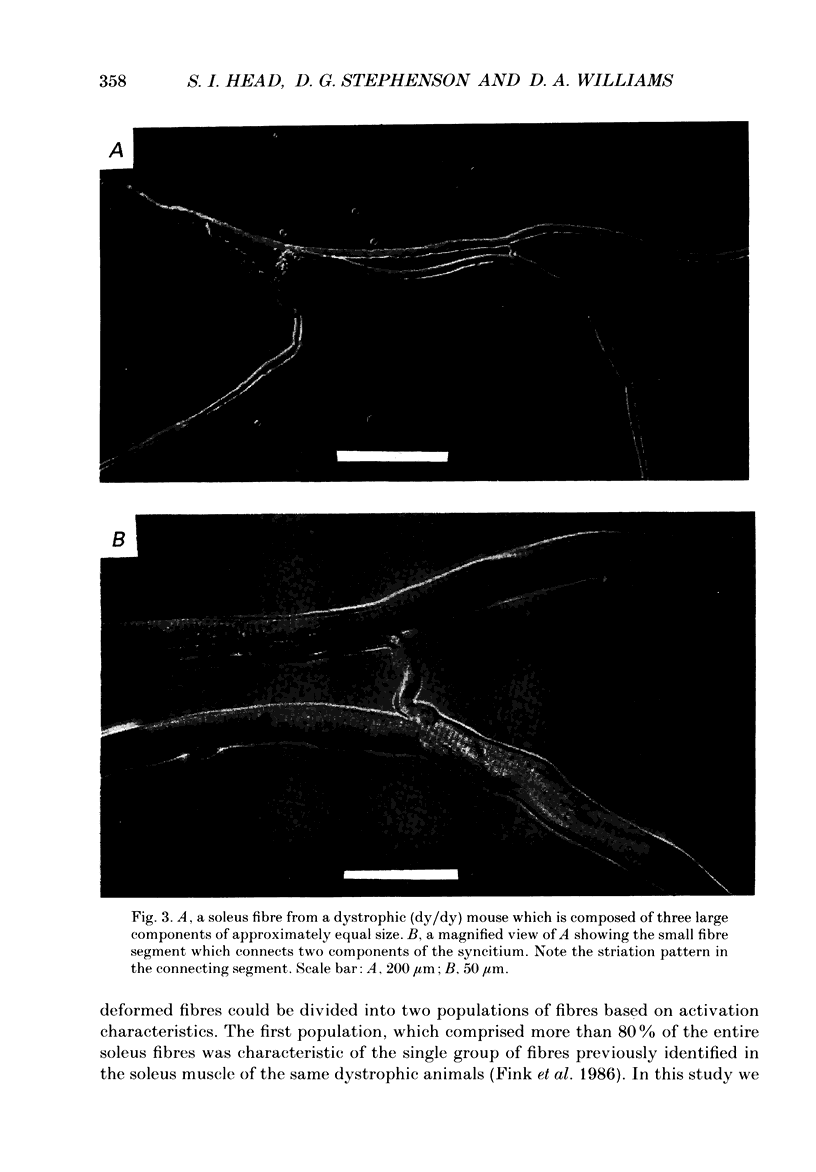
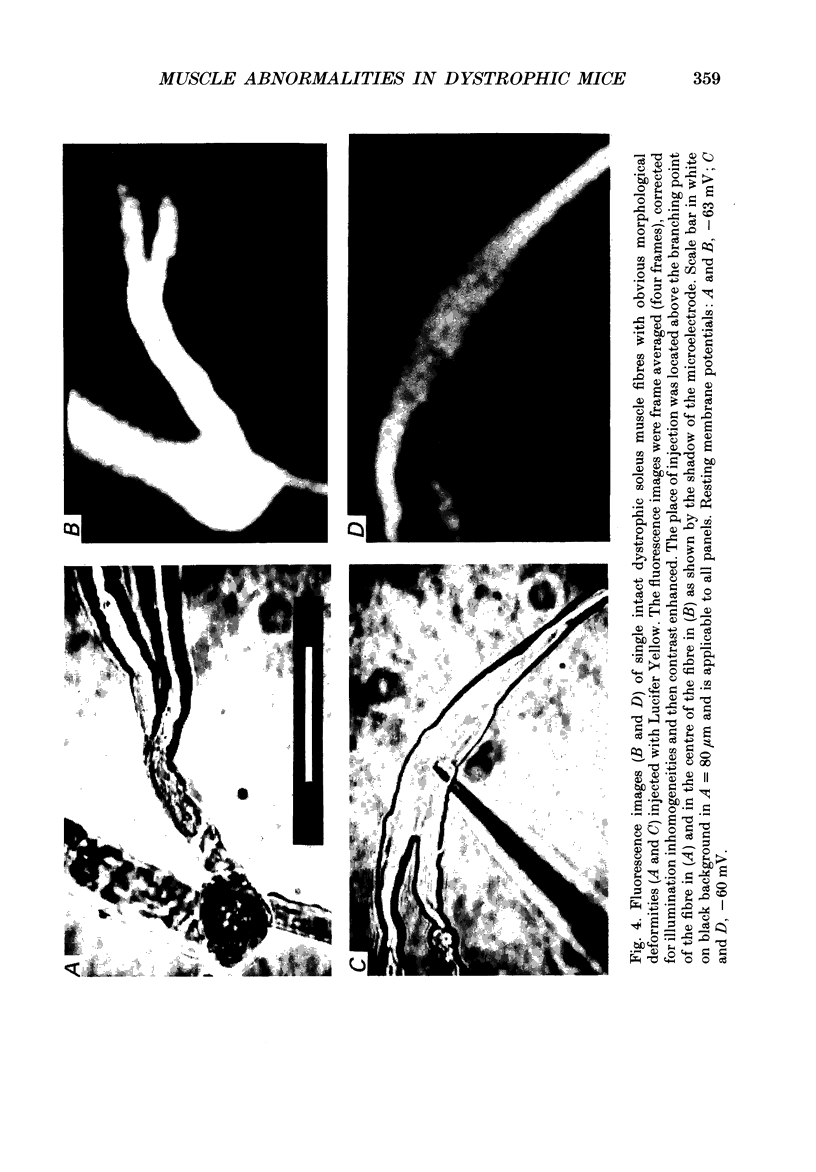
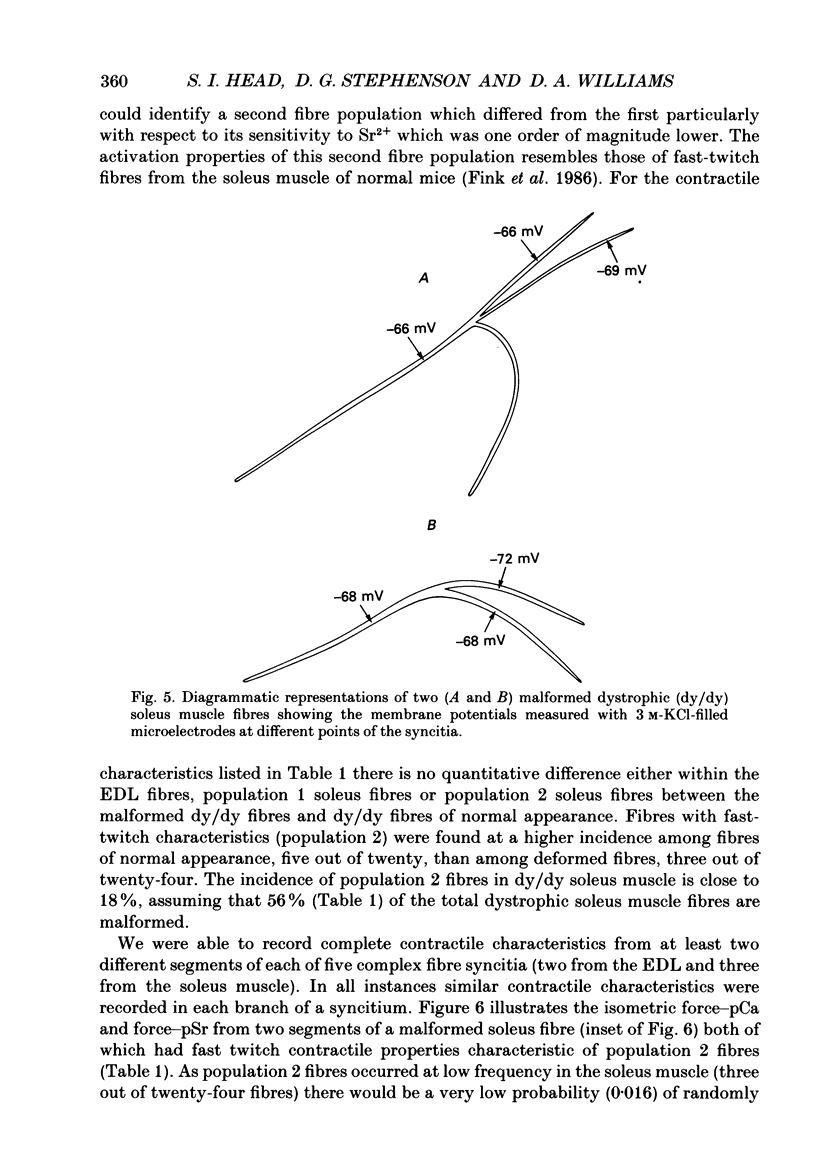
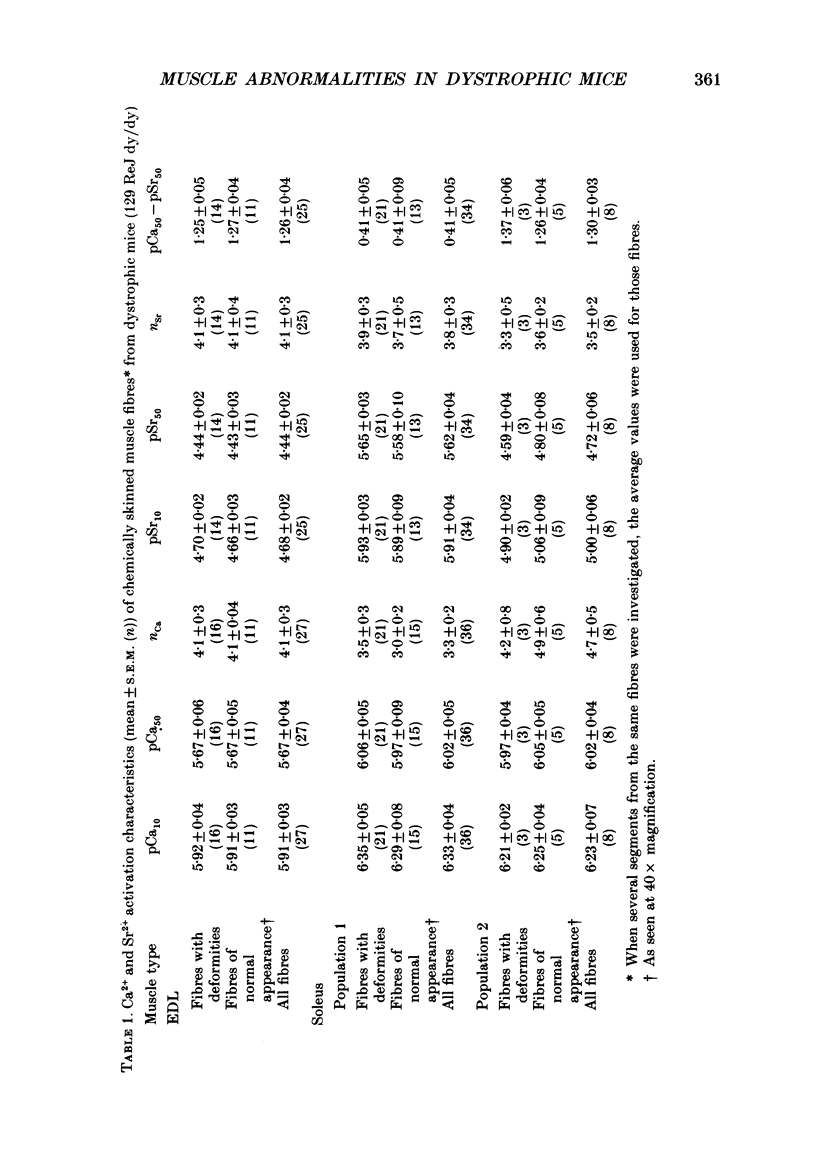
1. Single intact muscle fibres were enzymatically isolated from the skeletal muscles of the dystrophic mouse 129/ReJ dy/dy and were subjected to a range of physiological interventions. 2. Electrophysiological measurements, diffusion of injected dyes (Lucifer Yellow), microdissection and general appearance in the light microscope have shown that the majority of skeletal fibres isolated from the soleus and extensor digitorum longus (EDL) of adult dystrophic mice (10-14 weeks old) had gross morphological abnormalities. These abnormalities ranged from simple branching of the fibre to interconnections of many fibre branches which form a complex syncitium. 3. Segments from fibres of normal appearance and from fibres with morphological deformities were chemically skinned with Triton X-100 and activated in Ca2(+)- and Sr2(+)-buffered solutions. The different characteristics of the Ca2(+)- and Sr2(+)-activation curves were also used to identify the fibre type. 4. Gross morphological abnormalities were observed both in fibres which had predominantly slow-twitch and fast-twitch characteristics. 5. A new group of fibres was found to exist in the soleus muscle of dystrophic animals and represented about 18% of the entire soleus fibre population. This group of fibres had predominantly fast-twitch characteristics and some of these fibres were also grossly malformed. 6. The activation characteristics of individual branches from the same complex syncitium were similar, indicating that the contractile and regulatory proteins were of one type in one syncitium. 7. Chemically skinned segments from malformed fibres which included a major deformity between the points of attachment were generally unable to sustain near-maximal forces. 8. The proportion of malformed fibres which remained intact decreased markedly after prolonged tetanical stimulation of the intact muscle. This strongly suggests that malformed fibres are also functionally weak and prone to progressive damage when stimulated within the intact muscle. 9. The presence in large proportions of fibres with gross morphological abnormalities may explain the symptoms of severe and progressive muscle weakness and muscle loss which are apparent in the 129/ReJ dy/dy mice and possibly even in the human dystrophies such as Duchenne muscular dystrophy.
Full text
PDF




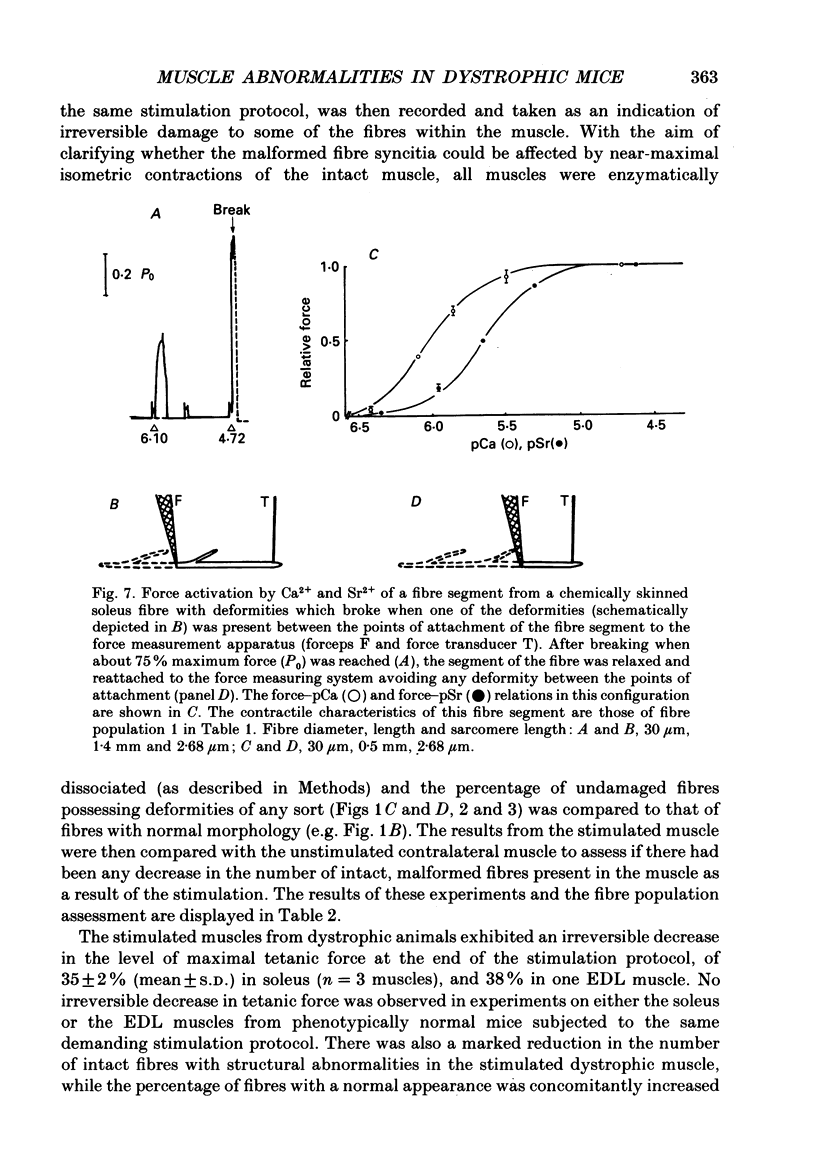



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bekoff A., Betz W. J. Physiological properties of dissociated muscle fibres obtained from innervated and denervated adult rat muscle. J Physiol. 1977 Sep;271(1):25–40. doi: 10.1113/jphysiol.1977.sp011988. [DOI] [PMC free article] [PubMed] [Google Scholar]
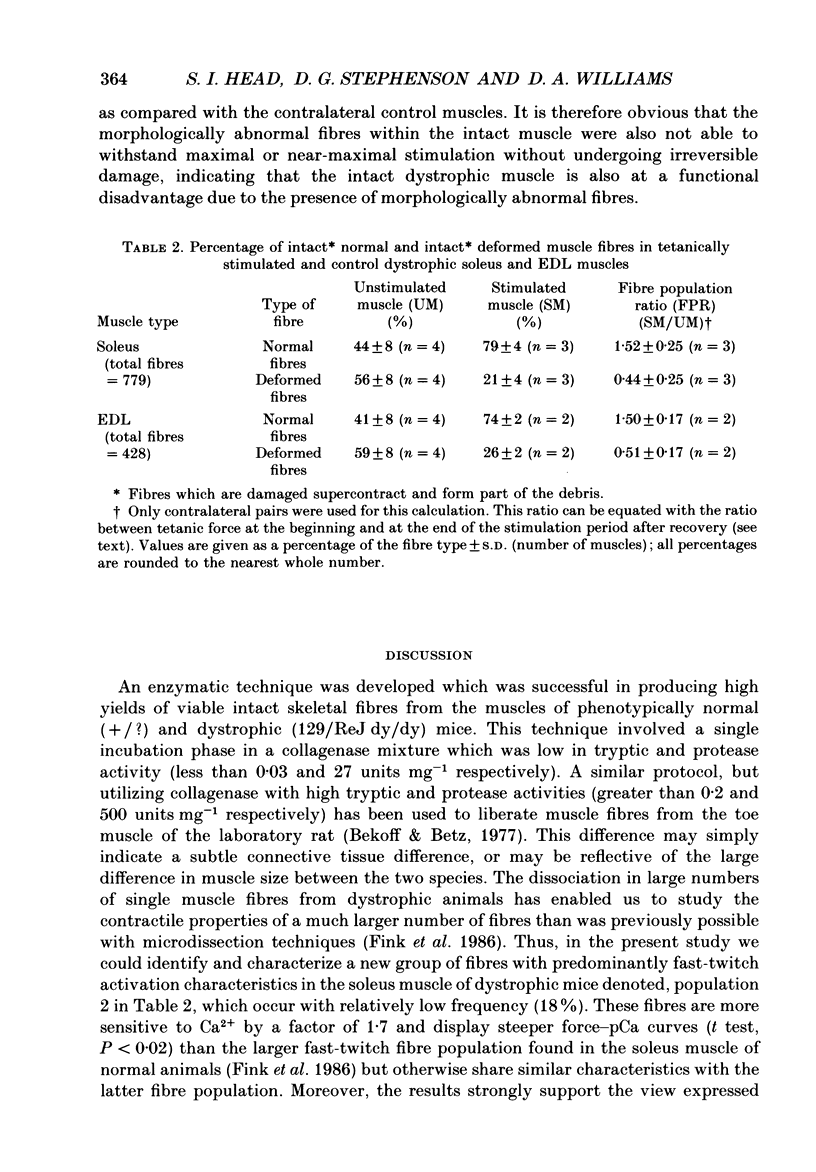
- Fink R. H., Stephenson D. G., Williams D. A. Calcium and strontium activation of single skinned muscle fibres of normal and dystrophic mice. J Physiol. 1986 Apr;373:513–525. doi: 10.1113/jphysiol.1986.sp016060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs E. R., Bradley W. G., Henderson G. Longitudinal fibre splitting in muscular dystrophy: a serial cinematographic study. J Neurol Neurosurg Psychiatry. 1973 Oct;36(5):813–819. doi: 10.1136/jnnp.36.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontell M., Feng K. C. The three-dimensional cytoarchitecture and pattern of motor innervation of branched striated myotubes. Anat Rec. 1981 May;200(1):11–31. doi: 10.1002/ar.1092000103. [DOI] [PubMed] [Google Scholar]
- Ontell M., Hughes D., Bourke D. Secondary myogenesis of normal muscle produces abnormal myotubes. Anat Rec. 1982 Nov;204(3):199–207. doi: 10.1002/ar.1092040304. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981 Aug;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. W. Lucifer dyes--highly fluorescent dyes for biological tracing. Nature. 1981 Jul 2;292(5818):17–21. doi: 10.1038/292017a0. [DOI] [PubMed] [Google Scholar]
- Webster C., Silberstein L., Hays A. P., Blau H. M. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell. 1988 Feb 26;52(4):503–513. doi: 10.1016/0092-8674(88)90463-1. [DOI] [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. The effect of denervation and dystrophy on the adaptation of sarcomere number to the functional length of the muscle in young and adult mice. J Anat. 1976 Nov;122(Pt 2):455–465. [PMC free article] [PubMed] [Google Scholar]