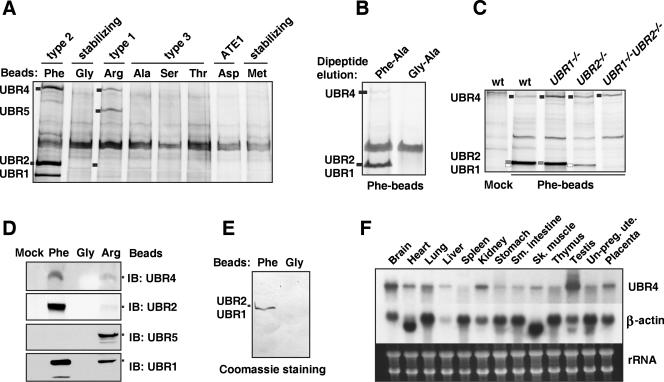
FIG. 2.
Identification of mouse N-recognins. (A) Peptide-pulldown assay using testis extracts and bead-conjugated peptides. Captured proteins were separated and visualized using silver staining. The identities of four major bands that are specifically and reproducibly captured by Phe- and/or Arg-peptide beads were determined using peptide mass fingerprinting. (B) UBR1, UBR2, and UBR4 bound from testis extract were eluted from Phe-peptide beads by the Phe-Ala dipeptide but not by Gly-Ala. (C) The amount of UBR4 bound by Phe-peptide beads from EF cell extracts does not depend strongly on the presence of UBR1 and/or UBR2. Proteins bound by either mock or Phe-peptide beads are indicated on the left with short bars: white, UBR1; gray, UBR2; and black, UBR4. (D) Testis proteins bound to bead-conjugated peptides bearing different N-terminal amino acids were immunoblotted with antibodies against UBR1, UBR2, UBR4, and UBR5. Mock, beads without peptide conjugation. (E) Semipurification of endogenous UBR1/UBR2 from an EF cytoplasmic extract using a peptide-pulldown assay. The precipitates/Phe-peptide beads complex prepared from cytoplasmic EF extracts were separated on SDS-PAGE and transferred onto a polyvinylidene difluoride membrane, followed by staining with Coomassie brilliant blue R-250 (Bio-Rad). Anti-UBR1 and -UBR2 immunoblotting confirmed enrichment of UBR1 and UBR2 (data not shown). (F) Northern blot analysis of UBR4 and β-actin using different mouse adult tissues. Total RNA (20 μg) was loaded in each lane. Ethidium bromide-stained ribosomal RNAs are shown as a loading control (bottom panel).