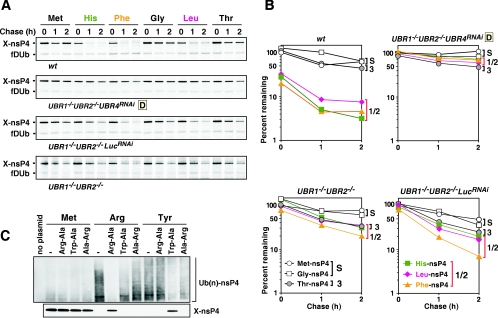
FIG. 6.
Degradation kinetics of X-nsP4 bearing N-terminal His, Phe, Gly, Leu, and Thr in various UBR mutant cells. (A) Pulse-chase analysis of X-nsp4, where X is Met or Gly (stabilizing), His (type 1 N-degron), Leu or Phe (type 2 N-degron), or Thr (type3 N-degron), in cells deficient in UBR proteins, singly or in combination. (B) Quantitation of the patterns shown in A using PhosphorImager. S, stabilizing; 1/2, type 1/2 N-degron; 3, type 3 N-degron. (C) Ubiquitylation assay of X-nsP4 in rabbit reticulocyte lysate. Upper panel: X-nsP4 proteins (where X is Met, Arg, or Tyr) were expressed and translationally labeled with biotin using transcription-translation-coupled reticulocyte lysates with or without 2 mM dipeptide as indicated. The proteasome inhibitor MG132 (5 μM) was also present, to accumulate ubiquitylated proteins. After 30 min of incubation, ubiquitylated proteins were immunoprecipitated with anti-Ub antibody, followed by SDS-PAGE of precipitated proteins and detection of biotinylated proteins (see Materials and Methods). Lower panel: X-nsP4 proteins (where X is Met, Arg, or Tyr) were in vitro translated as above without MG132 and a portion of the reticulocyte lysates was loaded, followed by Western blotting with horseradish peroxidase-conjugated streptavidin.