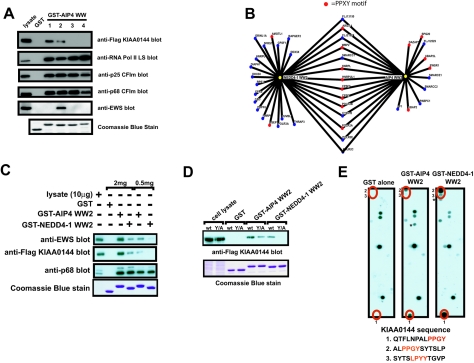
FIG. 8.
Specificity of binding between the different WW domains of AIP4 and the second WW domains of AIP4 and NEDD4-1. (A) 293T cells were transfected with a cDNA coding for a Flag-tagged KIAA0144 protein (FLAG-KIAA0144 blot) or left untransfected (other blots). Cell lysates were prepared, and an equivalent amount of lysate was precipitated with GST alone or with the indicated GST-AIP4 WW domain. Half of each precipitate was separated on an SDS-PAG, transferred to a PVDF membrane, and blotted with the indicated antibody. The other half of the GST pulldown was separated on an SDS-PAG and stained with Coomassie blue to show that equivalent amounts of GST fusion protein were used for the pulldowns (bottom blot). Cell lysate is included to show the electrophoretic mobilities of the indicated proteins. (B) Osprey diagram (7) showing the proteins identified by MS to precipitate with the second WW domains of NEDD4-1 and AIP4. Proteins with PY motifs are indicated in red. Note that the large subunit of RNA polymerase II (POL2A) does not have a PY motif but does possess several copies of the YSPTSPS heptamer sequence in its C-terminal domain (CTD), which has been shown to bind the WW domains of NEDD4 family proteins (12). (C) 293T cells were transfected with a cDNA coding for a Flag-tagged KIAA0144 protein (FLAG-KIAA0144 blot) or left untransfected (other blots). Cell lysates were prepared, and proteins were precipitated with the indicated GST fusion proteins from either 2 mg or 500 μg of cell lysate. Half of each precipitate was separated on an SDS-PAG, transferred to a PVDF membrane, and blotted with the indicated antibody. The other half of the GST pulldown was separated on an SDS-PAG and stained with Coomassie blue to show that equivalent amounts of GST fusion protein were used for the pulldowns (bottom blot). Cell lysate (10 μg) was included to show the electrophoretic mobility of the immunoblotted protein and to give an indication of the amount of protein precipitated by the GST fusion proteins. (D) 293T cells were transfected with a cDNA coding for a Flag-tagged KIAA0144 protein (wild type [wt]) or a Flag-tagged KIAA0144 protein in which the tyrosine residue in the PY motif had been mutated to alanine (Y/A). Cell lysates were prepared, and proteins were precipitated with the indicated fusion proteins from equivalent amounts of cell lysate. Half of each precipitate was separated on an SDS-PAG, transferred to a PVDF membrane, and blotted with the anti-FLAG M2 MAb. The other half of the GST pulldown was separated on an SDS-PAG and stained with Coomassie blue to show that equivalent amounts of GST fusion protein were used for the pulldowns (bottom blot). Cell lysate was included to show the electrophoretic mobility of the FLAG-KIAA0144 protein. (E) SPOTS membranes were prepared as outlined in Materials and Methods. Proteins (12-mers) comprising the entire coding sequence of KIAA0144 were generated, with a moving window of four amino acids. SPOTS membranes were probed with 1 μΜ of either GST-AIP4 WW2, GST-NEDD4-1 WW2, or GST alone. Spots specific to GST-AIP4 WW2 and NEDD4-1 WW2 are numbered and indicated in red. The sequences of these peptides (with the PY motif and LPXY motifs in red) are shown below the blots. Spots marked by an asterisk were recognized by GST alone in other experiments.