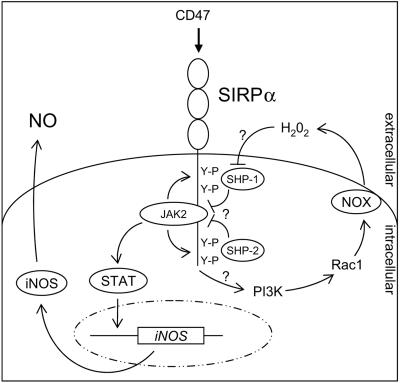
FIG. 8.
Model for the signaling events that mediate SIRPα-induced NO production in macrophages. SIRPα oligomerization by ligand triggers cross-phosphorylation of SIRPα-associated JAK2 and promotes SIRPα tyrosine phosphorylation, which then allows STAT docking and tyrosine phosphorylation. Activated STAT dimers induce iNOS gene expression and subsequent NO production. Simultaneously, SIRPα ligation triggers Rac1 activation, in a PI3-K-dependent fashion, followed by NOX assembly and oxidative burst generation. The resultant H2O2 inhibits the catalytic activity of SIRPα-associated SHP-1 and SHP-2, thereby preventing JAK2 and SIRPα dephosphorylation resulting in sustained JAK-STAT signaling and enhanced SHP-1 and SHP-2 retention by SIRPα.