FIG. 6.
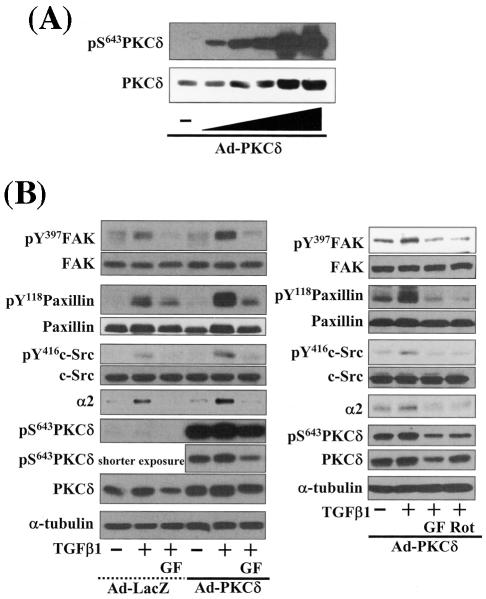
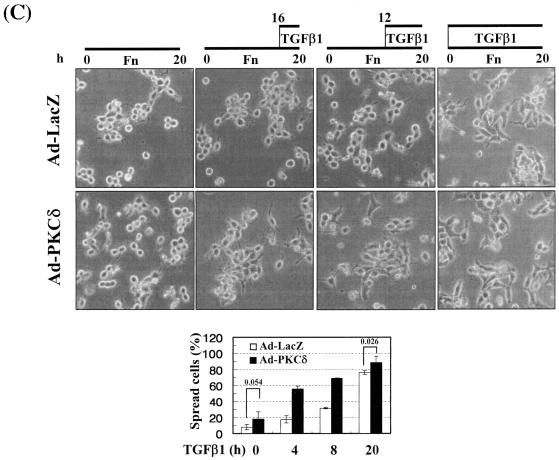
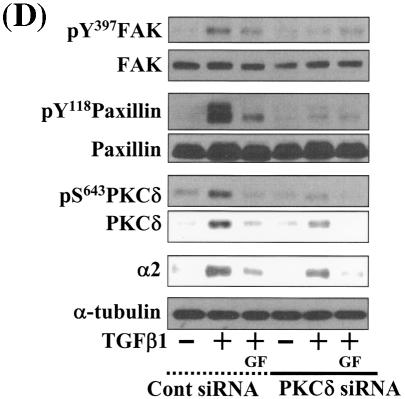
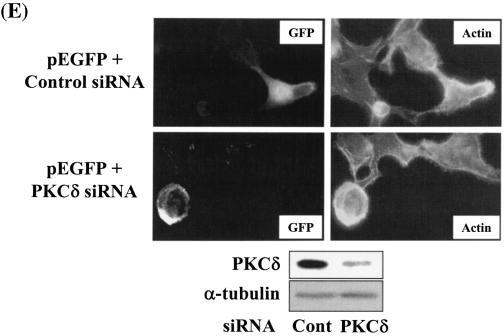
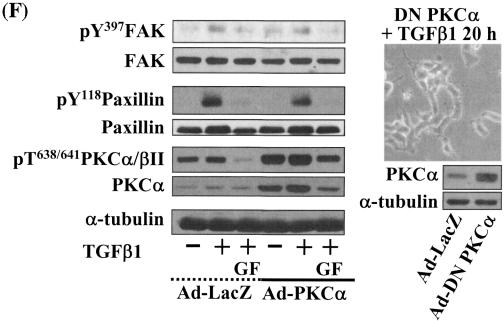
Integrin- and TGFβ1-mediated cell spreading requires PKCδ expression and activation. (A) Infection with adenovirus for PKCδ WT (Ad-PKCδ) increased the expression and Ser643 phosphorylation level of PKCδ. Cells were infected with various amounts of Ad-PKCδ. Two days later, cell lysates were prepared and used in Western blots for the indicated molecules. Data shown were representative of three independent experiments. (B) Increased activity and expression level of PKCδ by viral infection enhanced phosphorylation of the FA molecules. Cells were infected with a control adenoviral vector (Ad-LacZ) or Ad-PKCδ. Twenty-four hours later, cells were replated on Fn. Certain cells were pretreated with GF-109203X (GF) or Rottlerin (Rot), as described for Fig. 2. After the 20-h incubation on Fn, cell lysates were prepared and used in Western blots with antibodies against the indicated molecules. Data shown were representative of three different experiments. (C) Integrin- and TGFβ1-mediated cell spreading on Fn was accelerated by Ad-PKCδ infection. Cells infected with Ad-LacZ or Ad-PKCδ were replated on Fn with a concomitant TGFβ1 treatment for the indicated periods at the end of the 20-h incubation. Images were taken after the incubation on Fn. Quantitation of spread cells (cells with the longest distance from one end to the other end at least twice longer than the shortest distance) were counted from five isolated images of each experimental condition, and average values were graphed (mean ± standard deviation). A P value less than 0.05 from paired Student's t tests was considered significant. (D) Phosphorylation of the FA molecules was abolished by suppression of PKCδ protein. Cells were first transfected with siRNA to down-regulate PKCδ (PKCδ siRNA) or a control siRNA (Cont siRNA). Twenty-four hours after the transfection, cells were manipulated to be replated on Fn for the indicated experimental conditions. Cell lysates were then prepared and used for Western blots. Data shown represent three independent experiments. (E) Cell spreading was abolished by suppression of PKCδ protein. Cells were manipulated as explained for panel D, except that a part of the cells cotransfected with pcDNA3-GFP plus control siRNA or PKCδ siRNAwere replated on Fn-precoated cover glasses. Cells on cover glasses were processed for actin staining using phalloidin-conjugated TRITC. The other part of the cells was harvested after the 20-h incubation, and the lysates were immunoblotted using the indicated antibodies. Data shown were representative of three isolated experiments. (F) PKCα appeared not to be involved in the TGFβ1 effects. (Left) No enhancement of phosphorylation of the FA molecules upon PKCα overexpression. Cells were infected with either control virus (Ad-LacZ) or PKCα adenovirus (Ad-PKCα). One day after, cells were replated on Fn for the 20-h incubation under the indicated conditions. Lysates were prepared after the incubation and used in the immunoblots for the indicated molecules. (Right) Cells were infected with dominant-negative PKCα adenovirus (Ad-DN PKCα). Twenty-four hours later, cells were replated on Fn for the 20-h incubation in the presence of TGFβ1 treatment. After the incubation, the cell image was taken and then lysates were prepared for the immunoblots for the indicated molecules. Data shown were representative of 3 independent experiments.