FIG. 7.
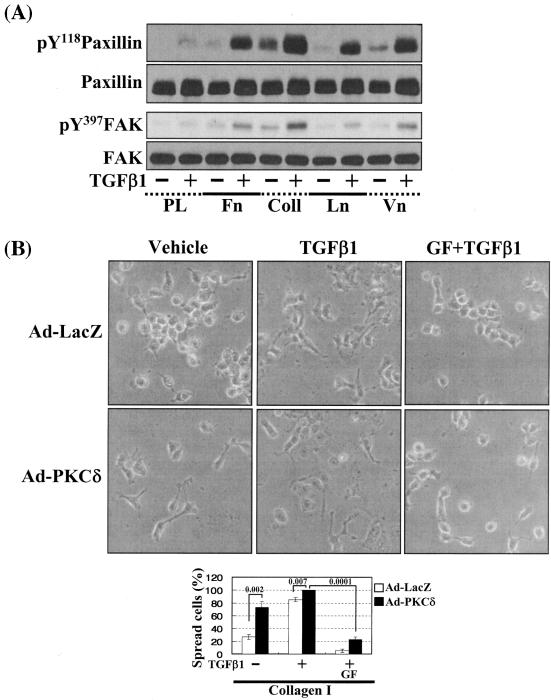
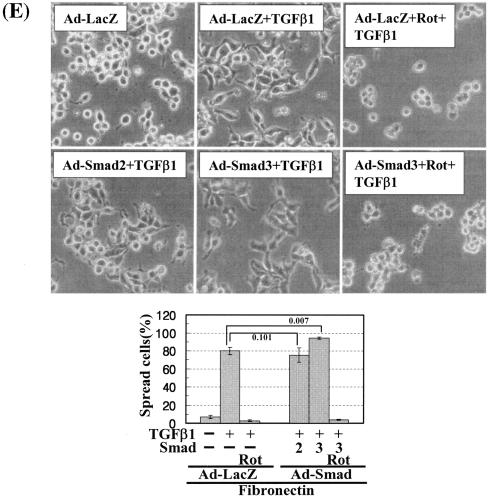
Cells under more efficient integrin-related signaling activities lead to enhanced cell spreading. (A) Phosphorylation of the FA molecules in cells replated on poly-l-lysine or diverse ECM proteins. Cells were replated on culture dishes precoated with various ECMs (10 μg/ml) (Coll, collagen I; Ln, laminin I; Vn, vitronectin), in the absence or presence of TGFβ1 treatment for 20 h. Cell lysates were then prepared and used for Western blots with antibodies against the indicated molecules. Data shown represent three independent experiments. (B) Spreading of PKCδ WT-overexpressing cells on collagen I even without TGFβ1 treatment. Twenty-four hours after infection with Ad-LacZ or Ad-PKCδ, cells were manipulated to be replated on collagen I-precoated dishes. Certain cells were pretreated with GF-109203X (GF), as described above. Twenty hours after the replating, images were taken. The rate of spread cells in percent graphed was determined as explained for Fig. 6C. A P value of ≤0.05 was considered significant. (C) Cells infected with Ad-PKCδ enhanced basal and TGFβ1-mediated expression of integrin α2 and phosphorylation of the FA molecules on collagen I. Cell manipulation and cell lysate preparation were performed, as explained above. Data shown were representative of three independent experiments. (D, E) Smad3 overexpression enhanced TGFβ1-mediated phosphorylation of the FA molecules (D) and cell spreading (E). Cells were infected with adenovirus encoding for either control (Ad-LacZ), Flag-Smad2 (Ad-Smad2), or Flag-Smad3 (Ad-Smad3). Twenty-four hours later, infected cells were replated on Fn. In certain cases, GF-109203X or Rottlerin (Rot) pretreatment was done as explained above. After 20 h of incubation at 37°C, cell lysates were prepared for Western blots, or cell images were taken. Quantitative determinations of the spread cells were done as explained for Fig. 6C. A P value of ≤0.05 was considered significant. Data shown were representative of three isolated experiments.