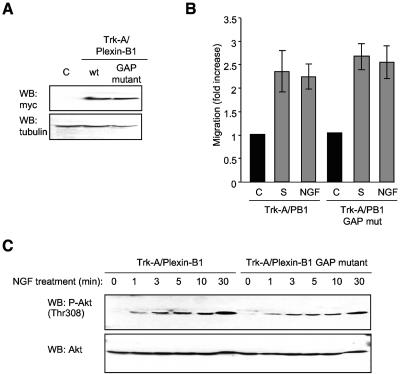
FIG. 3.
Endothelial cell migration and Akt phosphorylation and activation in response to Semaphorin 4D occur independently of residues involved in Plexin-B1 Ras GAP activity. (A) Transfected cells show immunoreactivity for the Ras GAP mutant form of the chimera Trk-A/Plexin-B1 (GAP mutant), at levels similar to that seen in cells expressing the wild-type intracellular portion of Plexin-B1 fused to Trk-A (wt, upper panel). Empty vector transfected cells were used as a negative control (C) and tubulin as the loading control (WB: tubulin, lower panel). (B) Endothelial cells expressing Trk-A/Plexin-B1 or Trk-A/Plexin-B1 Ras GAP mutant chimeras were used in a cell migration assay with 100 ng/ml NGF as the chemoattractant (NGF). Media containing 10% fetal bovine serum (S) was used as a positive control for migration. The bars represent the fold increase of migration as determined by densitometry relative to that seen in negative control wells containing 0.1% BSA (C). (C) Cells expressing Trk-A/Plexin-B1 or Trk-A/Plexin-B1 Ras GAP mutant chimeras were treated with 200 ng/ml Semaphorin 4D for the indicated periods of time, lysed, and analyzed for the presence of phosphorylated Akt. Phospho-Akt (WB: P-Akt [Thr308]) was seen in response to NGF treatment of Trk-A/Plexin-B1 expressing cells and in those expressing the Ras GAP mutant (upper panel). Total Akt was used as a loading control (WB: Akt, lower panel). mut, mutant.