FIG. 4.
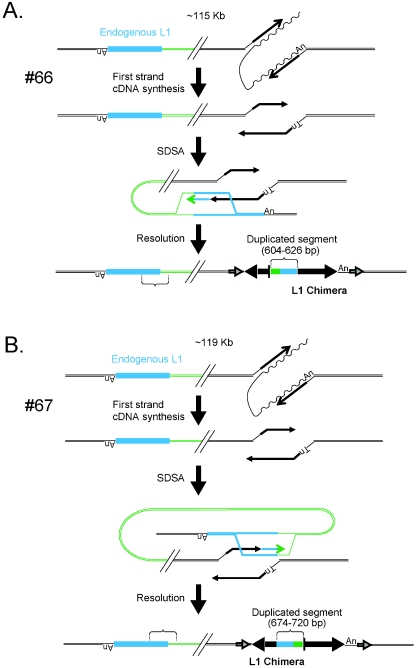
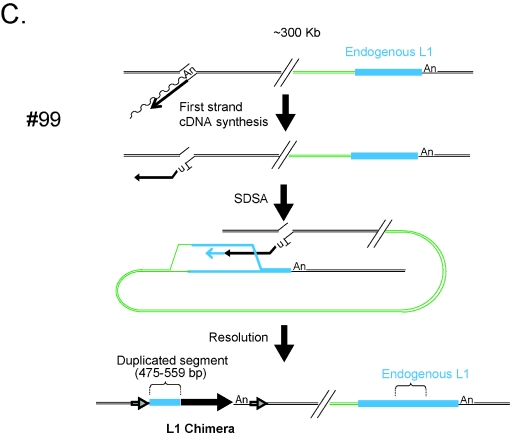
Chimeric L1 insertions associated with genomic duplications. Insertions 66, 67, and 99 resulted in the formation of chimeric L1s and the duplication of an intrachromosomal segment of DNA. Each insertion initiated by TPRT and likely was repaired by synthesis-dependent strand annealing. The undulating and straight black lines represent L1 mRNA and minus-strand L1 cDNA, respectively. In event 66 (A), twin priming resulted in cDNA synthesis using the 3′-OH present at the top and bottom strands of the target site. The bottom-strand cDNA then used an endogenous L1 located ∼115 kb upstream of the insertion site (light blue rectangle) as a template for SDSA. As a result the newly integrated L1 was joined to the endogenous L1 as well as its flanking DNA (green line). Resolution of the intermediate resulted in the inversion/duplication of a 604- to 626-bp segment of DNA and the formation of a chimeric L1. The entire insertion is flanked by a 15-bp TSD (indicated as in Fig. 1). In event 67 (B), twin priming resulted in two L1 cDNAs using the 3′-OH present at the top and bottom strands of the target site. The top-strand cDNA then used an endogenous L1 located ∼119 kb upstream of the insertion site (light blue rectangle) as a template for SDSA. As a result the newly integrated L1 cDNA was covalently joined to the endogenous L1 as well as its flanking DNA (green line). Resolution of the intermediate resulted in the duplication of a 674- to 720-bp segment of DNA and the formation of a chimeric L1. The entire insertion is flanked by a 14-bp TSD (indicated as in Fig. 1). In event 99 (C), TPRT resulted in the initiation of L1 cDNA synthesis. The resultant cDNA then used an endogenous L1 located ∼300 kb downstream of the insertion site (light blue rectangle) as a template for SDSA. As a result the newly integrated L1 cDNA was covalently joined to the endogenous L1 (green line). Resolution of the intermediate resulted in the duplication of a 475- to 559-bp segment of L1 DNA and the formation of a chimeric L1. The entire insertion is flanked by a 159-bp TSD (indicated as in Fig. 1). The large horizontal black arrows indicate the transcriptional orientation of new inserted L1 fragments. The vertical bar is a schematic of the junction of the inverted fragments. In the three cases, the presence of discriminating single nucleotide polymorphisms between the engineered L1 and the endogenous L1 was used to determine the size ranges of the duplicated L1 fragments.