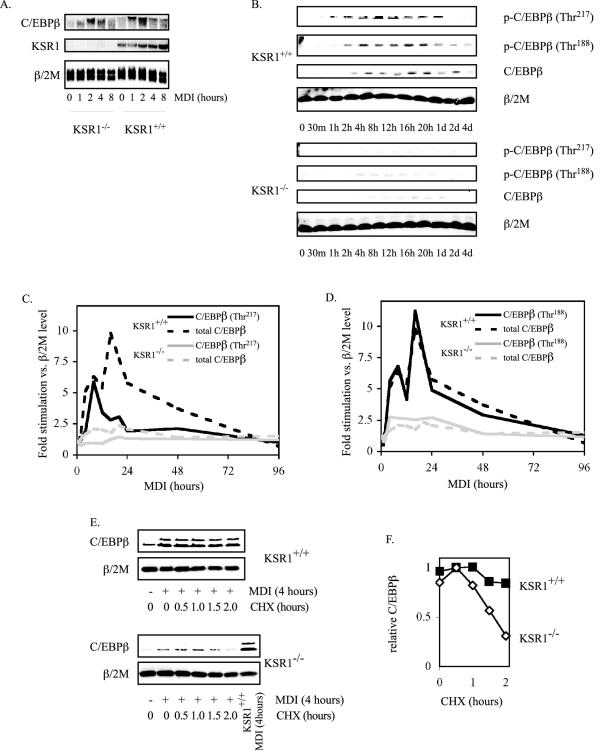
FIG. 4.
KSR1 is required for C/EBPβ phosphorylation and stabilization during adipogenesis. (A) MDI-induced C/EBPβ transcription is independent of KSR1 expression. Shown is Northern blot analysis of C/EBPβ, KSR1, and β/2 M at different times after induction of differentiation in KSR1−/− and KSR1+/+ MEFs. (B) KSR1 enhances C/EBPβ phosphorylation during adipogenesis. Shown is Western blot analysis of whole-cell extracts prepared at different times during differentiation of KSR1−/− and KSR1+/+ MEFs. Lysates were probed with the indicated antibodies to detect induction and activation of C/EBPβ during adipogenesis. (C and D) Total and phosphorylated C/EBPβ expression was quantified from the Western blots described above (B) at each time point and normalized to β/2 M levels, and the resulting data were plotted as fold stimulation following induction of differentiation. Data for Thr217 phosphorylation are shown in C, and data for Thr188 phosphorylation are shown in D. KSR1+/+, black line; KSR1−/−, gray line; phospho-C/EBPβ, solid line; total C/EBPβ, dashed line. Data shown in each panel are the averages of three independent experiments. (E and F) Effect of KSR1 on C/EBPβ stability. Shown is Western blot analysis of whole-cell extracts prepared from KSR1−/− and KSR1+/+ MEFs after 4 h of induction with differentiation medium followed by 0 to 2 h of treatment with cycloheximide (CHX). Lysates were probed for C/EBPβ and β/2 M. C/EBPβ expression at each time point was quantified and normalized to β/2 M levels, and the resulting data were plotted in F. Maximal expression was set arbitrarily to 1. Values are the means of two independent experiments.