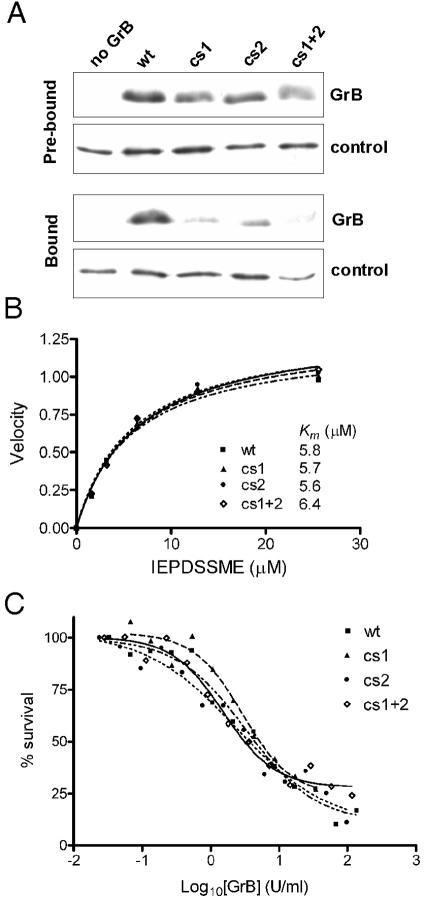
FIG. 4.
GrB cs mutants have impaired GAG binding but normal catalytic and apoptotic function. (A) Heparin-binding of cs mutants. Equal amounts of wt or mutant GrB were mixed with heparin-Sepharose beads. An irrelevant heparin-binding control protein (antithrombin) was included as a tracer to monitor loss of beads during the procedure. Samples were assessed by SDS-PAGE and immunoblotting. The Prebound panel compares the input GrBs used in the experiment, with no binding or washing steps, as assessed immediately after mixing with the tracer. The Bound panel shows the GrB remaining after binding and washing. Results are representative of five independent experiments. (B) The catalytic activity of wt and cs mutants on a quenched-fluorescence substrate Abz-IEPDSSMESK-dnp was measured as described previously (46). (C) The apoptotic function of the cs mutants was assessed using a modified in vitro killing assay, in which perforin was replaced by SLO. Enzymatic activities of the granzymes were matched via IETD-pNA cleavage assay. Jurkat cells were incubated for 1 h at 37°C with an empirically determined amount of SLO (permeabilizes 80% of cells) and increasing concentrations of GrB. Cells were washed, and survival was assessed 16 h later by MTT assay. The EC50 for wt GrB was 5 U/ml.