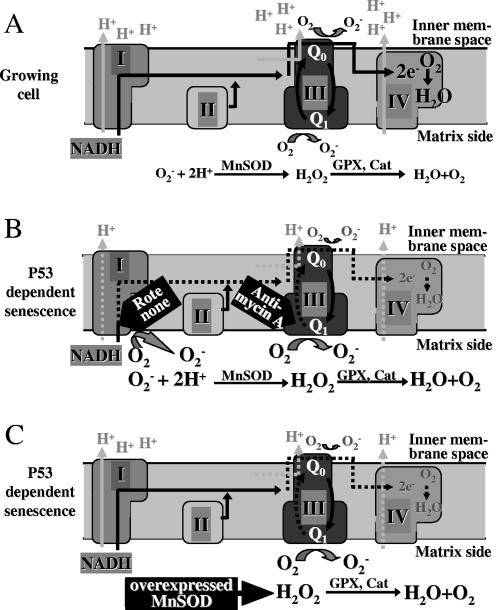
FIG. 8.
Model for the reduction of MMP by MnSOD overexpression. MnSOD acts as an electron trap on the matrix side. (A) Mechanistic view of the electron transport chain of the inner mitochondrial membrane of normally growing cells. Electrons are transported from reduced substrates (such as NADH) along the complexes of the respiratory chain of the inner mitochondrial membrane onto oxygen in complex IV. By simultaneous transport of protons from the matrix to the inner membrane space, an electrochemical force is generated called mitochondrial membrane potential, which drives ATP synthesis. As by-products, electrons are transferred onto oxygen primarily at the level of complex III, leading to superoxide radical production in the inner membrane space (from the cytochrome Q0 site) and at the matrix side (cytochrome Q1 site). The enzymes of the antioxidant defense system in the mitochondrial matrix, such as MnSOD, catalase (Cat), and glutathione peroxidases (GPX), detoxify superoxide radicals to water and oxygen. (B) Uncoupling of electron transport by ROT at the level of complex I or by AA at the level of complex III generates increased amounts of electrons at the matrix side of the mitochondrion, which leads to enhanced matrix and reduced cytosolic superoxide production. The dysfunctional electron transport impairs the proton transport, which diminishes the MMP. (C) We assume that loss of MMP is also induced by MnSOD overexpression via intensified electron consumption in the matrix of the mitochondrion. Presumably, at the Q1 site of complex III, electrons flow into the increased dismutation reaction catalyzed by elevated levels of MnSOD. Because of the reduced electron flow to the inner membrane space, cytosolic superoxide levels are reduced. Consequently, MnSOD acts as a kind of electron trap on the mitochondrial matrix side, which redirects further electrons to the matrix side, thereby increasing the production of hydrogen peroxide, which is detoxified by GPX and Cat, eventually leading to a lack of electrons and to membrane depolarization.