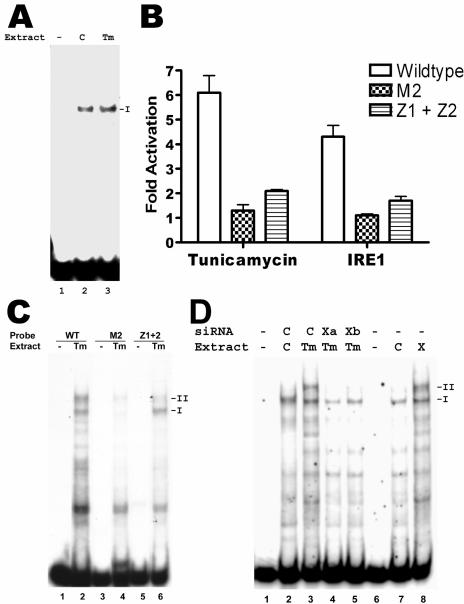
FIG. 7.
Dependence of complex II formation on both CCAAT element and CT-rich elements of the S promoter, as well as XBP1(s). A. EMSA of CCAAT element of the S promoter, using nuclear extracts from tunicamycin-treated (Tm) or dimethyl sulfoxide-treated control (C) HuH7 cells. Both extracts gave rise to only one shifted band (I), corresponding to binding by NF-Y. B. Activation of wild-type or mutant S promoters by tunicamycin and IRE1-alpha. On the left side, CAT reporter plasmids driven by the wild-type S promoter, or mutated S promoters with lesions in the CCAAT element (M2) or the CT-rich elements (Z1+Z2), were transfected into HuH7 cells, and one set of cells was treated with tunicamycin for 17 h before harvesting. The CAT activity in these cells was normalized to that in cells treated with dimethyl sulfoxide. On the right side, HuH7 cells were cotransfected with the indicated S promoter reporter plasmid and the plasmid expressing IRE1-alpha, and the CAT activity was normalized to that in cells cotransfected with the empty vector. C. EMSA of wild-type or mutated S promoters, using extracts from tunicamycin-treated HuH7 cells. Mutation of the CCAAT element (M2) prevented formation of both complexes I and II, while mutation of the CT-rich elements (Z1+Z2) prevented formation of only complex II. D. Lanes 1 to 5: EMSA of wild-type S promoter, using extracts from HuH7 cells treated with tunicamycin (Tm; lanes 3, 4, and 5) or dimethyl sulfoxide (C; lane 2) and transfected with two different siRNAs against XBP1 (Xa and Xb; lanes 4 and 5, respectively) or a control siRNA (C; lanes 2 and 3). Lanes 6 to 8: EMSA of wild-type S promoter, using extracts from HuH7 cells transfected with a plasmid expressing XBP1(s) (X; lane 8) or the empty vector (C; lane 7). No extracts were used in lanes 1 and 6.