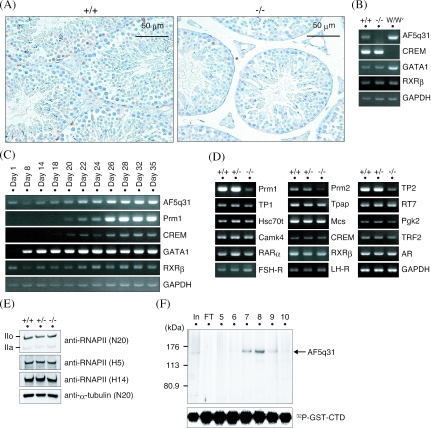
FIG. 6.
Mechanism of defective spermatogenesis in AF5q31-deficient mice. (A) Expression of AF5q31 in testes. Immunohistochemical staining was performed with an anti-mAF5q31-E4 antibody on sections of the testes from 12-week-old AF5q31+/+ and AF5q31−/− mice. Sections were counterstained with hematoxylin. Brown areas represent the positive signals. (B) RT-PCR analyses of AF5q31 expression using total RNAs isolated from the testes of 12-week-old AF5q31+/+ and AF5q31−/− male mice and 9-week-old W/Wv male mice. RT-PCR for GAPDH confirms the equivalent amounts of RNAs used for the analysis. (C) Expression of AF5q31 during juvenile testis development in mice. RT-PCR analyses of AF5q31 exons V to VIII and several marker genes in testis are demonstrated. RT-PCR for GAPDH confirms the equivalent amounts of RNAs used for the analysis. (D) Expression of spermatogenesis- and spermiogenesis-related genes in the testes of 12-week-old AF5q31+/+, AF5q31+/−, and AF5q31−/− male mice. RT-PCR for GAPDH confirms the equivalent amounts of RNAs used for the analysis. (E) RNAPII CTD phosphorylation in AF5q31+/+, AF5q31+/−, and AF5q31−/− MEFs. Whole-cell extracts (10 μg/lane) were immunoblotted with the indicated antibodies. As a control, anti-α-tubulin was used to monitor the loading amounts. (F) In vitro kinase assay of P-TEFb in the presence or absence of AF5q31. Chromatography of purified HA-AF5q31-Flag on a Mono Q column revealed the presence of full-length AF5q31 (140 kDa). Each fraction (4 μl) on the Mono Q column was analyzed by SDS-PAGE and silver staining (upper panel). The lane marked “In” represents a part of the material before loading the column, and the lane marked “FT” indicates the flowthrough of the Mono Q column. Equal aliquots from each fraction were added to the kinase reaction mixture containing P-TEFb and GST-CTD and resolved by SDS-PAGE. Phosphorylated GST-CTD was detected by autoradiography (lower panel).