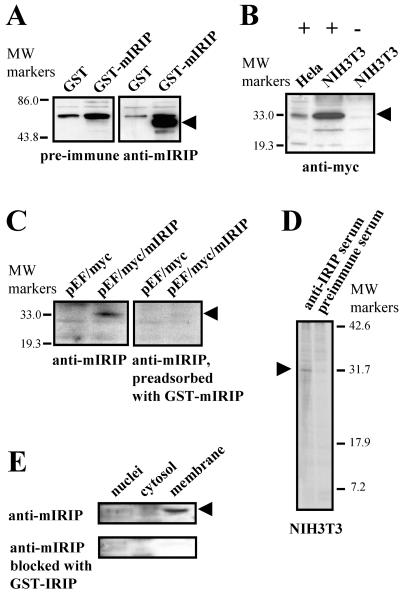
FIG. 7.
Generation of anti-IRIP antibiotics and expression of IRIP in cell lines and kidney subcellular fractions. (A) Purified mIRIP protein expressed in E. coli was used for generation of rabbit polyclonal antibodies. The purified GST-mIRIP was detected by antibodies in Western blots. (B) Cellular extracts were prepared from HeLa and NIH 3T3 cells transiently transfected with myc-tagged mIRIP construct (+) or cells transfected with expression vector (−). The recombinant protein was detected only in cells transfected with mIRIP. (C) Specificity of antibodies was also confirmed by testing cellular extracts from myc-tagged mIRIP (pEF/myc/mIRIP)-transfected cells with antibodies which were preadsorbed with GST-mIRIP. After blocking, mIRIP was not detected; its position is shown by an arrowhead. (D) Immunoprecipitation of endogenous IRIP with polyclonal rabbit antibodies. Mouse NIH 3T3 cells were metabolically labeled with [35S]methionine and [35S]cysteine. Total cell extracts were prepared and immunoprecipitated with either preimmune or anti-mIRIP antibodies. The precipitates were resolved on 15% SDS-PAGE gel. The ∼30-kDa bands, visible only in samples immunoprecipitated with the antiserum, are indicated with arrows. (E) Intracellular distribution was tested in subcellular fractions from mouse kidney. Cytosolic, nuclear, and plasma membrane fraction extracts were used for Western blot analysis with anti-mIRIP antibodies. The lowest blot shows that there was no labeling in any of the samples after blocking antibodies with the purified GST-IRIP fusion protein.