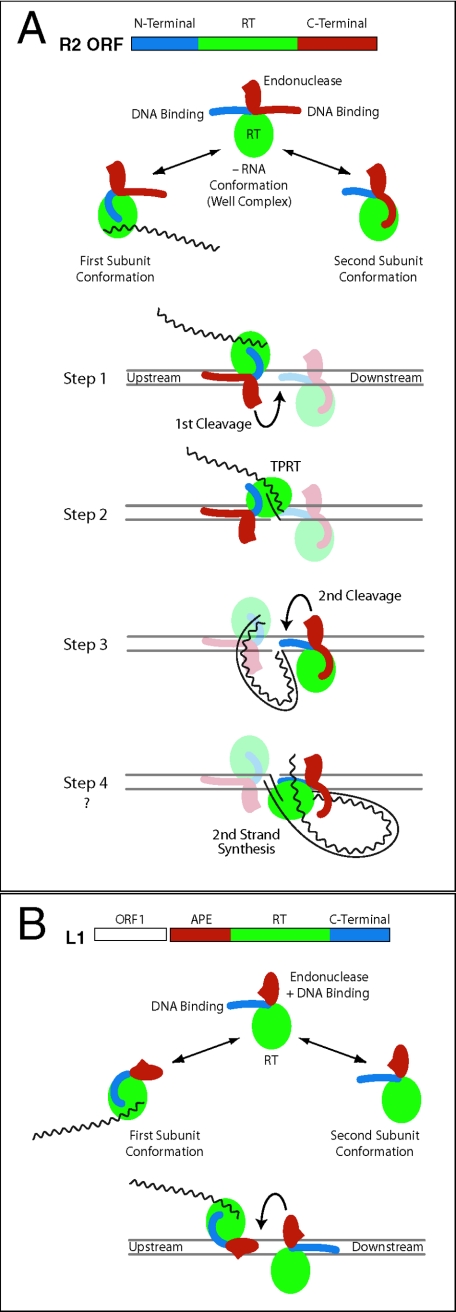
FIG.7.
Model of a complete R2 integration reaction. (A) The R2 ORF is divided into three domains: N-terminal DNA binding (blue), reverse transcriptase (green), and C terminal (red) (5). The C-terminal domain has been subdivided into DNA-binding and endonuclease subdomains. The DNA cleavage domain (red oval with spike) is on a flexible linker, similar to the type IIs restriction endonucleases. In the context of a dimer, each R2 subunit exposes only one DNA binding domain, while the other domain is sequestered. The subunit with bound 3′-UTR RNA exposes the C-terminal DNA-binding domain and binds upstream of the cleavage/integration site. The subunit lacking RNA exposes the N-terminal DNA binding domain and binds in opposite orientation downstream of the site. While it is likely that protein-protein interactions play a role in dimer formation, such interactions have not been depicted. Step 1 (first-strand cleavage). The EN domain of the upstream subunit cleaves the bottom strand. Step 2 (first-strand synthesis). The upstream subunit undergoes a conformational change, placing its reverse transcriptase over the bottom strand cut, which can then catalyze TPRT. Step 3 (second-strand cleavage). The EN domain of the downstream subunit cleaves the top strand. Step 4 (second-strand synthesis). The upstream subunit undergoes a conformational change, placing its RT over the top-strand cut, which can then catalyze second-strand synthesis. This last step does not occur in our in vitro reaction. (B) Model of L1 integration. L1 elements encode two ORFs (27). The second ORF contains a N-terminal AP endonuclease domain (red), a reverse transcriptase domain (green), and a C-terminal domain, which is postulated in the model to be DNA binding (blue). As in the R2 model, the active complex is assumed to be a dimer, with each subunit in opposite orientation conducting one-half of the reactions. One subunit binds RNA, binds the DNA target by means of the APE domain, cleaves the first strand, and conducts TPRT. The second subunit binds by means of the C-terminal domain, cleaves the second strand, and conducts second-strand DNA synthesis. No evidence yet exists for the L1 C-terminal domain binding DNA.