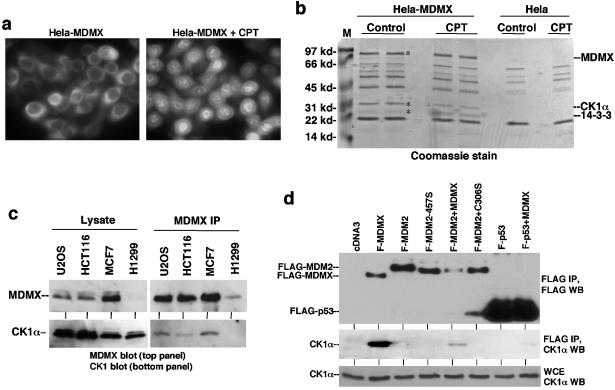
FIG. 1.
CK1α forms a complex with MDMX. (a) HeLa cells stably transfected with FLAG-tagged human MDMX were stained using anti-FLAG M2 antibody before or after treatment with 0.5 μM camptothecin (CPT) for 16 h. (b) Coomassie blue staining of affinity-purified MDMX and associated proteins. The two marked bands were identified as CK1α and 14-3-3τ by mass spectrometric peptide sequencing. Control purification was performed using HeLa cells to exclude background. The positions of molecular mass markers (M) (in kilodaltons) are shown to the left of the gel. (c) Cell lines expressing different levels of endogenous MDMX were analyzed by MDMX IP/CK1α Western blotting. The relative expression levels of MDMX and CK1α were determined by direct Western blotting of identical amounts of whole-cell extract from each cell line. (d) H1299 cells transfected with FLAG-tagged MDMX (F-MDMX), F-MDM2, and F-p53-281G mutant were analyzed by anti-FLAG IP and anti-CK1α Western blotting (WT) to detect coprecipitation of endogenous CK1α. Cotransfection of nontagged MDMX was tested for mediating formation of trimeric complexes. F-MDM2-457S is a RING domain mutant for control. C306S is an MDMX zinc finger mutant defective for CK1α binding. WCE, whole-cell extract.