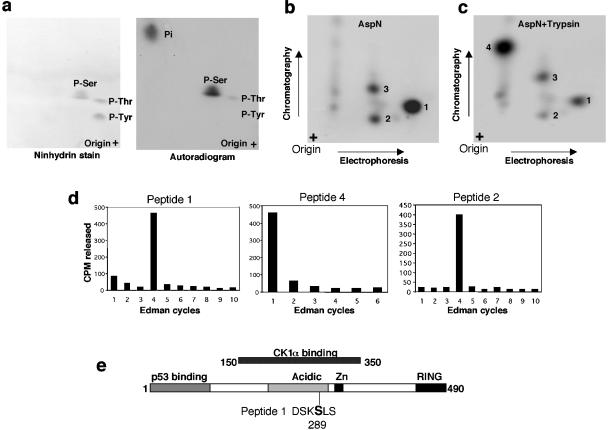
FIG. 6.
Identification of in vitro CK1α phosphorylation site on MDMX. (a) His6-MDMX phosphorylated by GST-CK1α and [γ-32P]ATP in vitro was hydrolyzed by HCl, and phosphoamino acids were analyzed by two-dimensional electrophoresis. Ninhydrin stain indicates the positions of phosphoamino acid standards. P-Ser, phosphorylated serine. (b and c) His6-MDMX phosphorylated by GST-CK1α and [γ-32P]ATP was digested with Asp-N or Asp-N/trypsin. Phosphopeptides were analyzed by electrophoresis, followed by chromatography on a cellulose plate. (d) Phosphopeptides recovered from panels b and c were subjected to manual Edman degradation, and the release of radioactivity in each cycle was measured to determine the position of the phosphoamino acid from the N terminus. (e) Serine 289 was identified as the major CK1α phosphorylation site on the basis of Edman degradation results and sequence inspection.