Abstract
Stem cell factor (SCF), erythropoietin (Epo), and GATA-1 play an essential role(s) in erythroid development. We examined how these proteins interact functionally in G1E cells, a GATA-1− erythroblast line that proliferates in an SCF-dependent fashion and, upon restoration of GATA-1 function, undergoes GATA-1 proliferation arrest and Epo-dependent terminal maturation. We show that SCF-induced cell cycle progression is mediated via activation of the Src kinase/c-Myc pathway. Restoration of GATA-1 activity induced G1 cell cycle arrest coincident with repression of c-Kit and its downstream effectors Vav1, Rac1, and Akt. Sustained expression of each of these individual signaling components inhibited GATA-1-induced cell cycle arrest to various degrees but had no effects on the expression of GATA-1-regulated erythroid maturation markers. Chromatin immunoprecipitation analysis revealed that GATA-1 occupies a defined Kit gene regulatory element in vivo, suggesting a direct mechanism for gene repression. Hence, in addition to its well-established function as an activator of erythroid genes, GATA-1 also participates in a distinct genetic program that inhibits cell proliferation by repressing the expression of multiple components of the c-Kit signaling axis. Our findings reveal a novel aspect of molecular cross talk between essential transcriptional and cytokine signaling components of hematopoietic development.
Receptor tyrosine kinases (RTKs) trigger a multitude of cellular events, including proliferation, survival, differentiation, and migration. These functions are modulated in hematopoietic stem and progenitor cells by the essential RTK c-Kit (8, 11, 43). The expression of c-Kit is downregulated as progenitors mature to their respective lineages, with the exception of mast cells, which rely on c-Kit for survival, proliferation, and function throughout their life span (20). Unrestrained c-Kit activity contributes to several neoplastic disorders, including gastrointestinal stromal tumors (GIST), mastocytosis, and leukemia (5, 12, 21, 37, 46, 55). In GIST, somatic kinase-activating Kit mutations result in malignant transformation. In the hematopoietic system, similar activating Kit mutations occur in stem/progenitor cells and mast cells, causing mastocytosis and acute myelogenous leukemia, respectively (45, 54).
Mutant mice without c-Kit (dominant white spotting, or W, mutants) demonstrate severe deficiencies in erythroid development with reduced CFU-erythroid progenitors in the fetal liver and embryonic death from anemia at around day 16 of gestation (11, 57). Erythropoietin (Epo) receptor (Epo-R)-deficient mice demonstrate a similar decrease in CFU-erythroid progenitors and die of anemia between days 13 and 15 of gestation, suggesting that erythroid progenitors cannot survive, proliferate, or differentiate unless both the c-Kit and Epo-R signal transduction pathways are functional. Recent studies suggest that Epo and Epo-R interactions contribute to this process by preventing apoptosis through activation of the survival factor Bcl-xL as well as Akt (3, 17, 25, 34, 49, 51, 52, 68, 74). The role of c-Kit in erythropoiesis is less well understood. Studies suggest that this RTK stimulates the expansion of early committed erythroid precursors, but its downregulation is required for later stages of terminal maturation (53, 83). However, the intracellular mechanisms that regulate c-Kit during erythroid development are poorly defined.
In addition to cytokine signaling cascades, erythroid progenitors also require transcriptional programs for survival, differentiation, and cell cycle regulation. To this end, the transcription factor GATA-1 is essential for erythropoiesis (19, 59, 60). Disruption of the GATA-1 gene produces maturation arrest and apoptosis of committed erythroid precursors (19, 60, 80). GATA-1 is also required for the development of mast cells, eosinophils, and platelets (19, 31, 48, 59, 67, 87). In humans, germ line GATA-1 mutations cause congenital dyserythropoietic anemia, thalassemia, and thrombocytopenia (56, 88). Somatic GATA-1 mutations are associated with acute megakaryocytic leukemia in the context of Down's Syndrome (85). Hence, an understanding of how GATA-1 coordinates erythroid cell differentiation, survival, and cell cycle progression is relevant to both normal hematopoietic development and human disease.
The purpose of the current study was to investigate whether GATA-1 influences c-Kit signaling during erythroid maturation. To examine this, we utilized G1E cells, an immortalized GATA-1-null line derived from gene-targeted embryonic stem cells (81). G1E cells proliferate continuously in culture as developmentally arrested erythroid precursors and, upon restoration of GATA-1 activity, undergo cell cycle arrest and terminal maturation in a fashion that largely recapitulates normal erythropoiesis (25, 34). In this regard, stem cell factor (SCF) and Epo are required for the growth and survival of these cells at distinct developmental stages. Specifically, immature G1E cells depend mainly upon SCF for proliferation. During GATA-1-induced maturation, c-Kit is downregulated and Epo becomes an essential survival factor (34, 61, 82). This sequence recapitulates cytokine requirements during normal erythroid maturation. Therefore, G1E cells provide a convenient, physiologically relevant system to delineate how GATA-1 regulates the dynamics of SCF and Epo cytokine signaling during erythropoiesis.
Previously, we used G1E cells to identify an important role for GATA-1 in regulating core cell cycle components by repressing the expression of mitogenic genes and activating antiproliferative ones (61, 82). Of particular relevance to the current study, GATA-1 appears to repress c-Myc expression by directly binding its gene (61). Here, we demonstrate that without GATA-1, immature G1E cells depend on SCF for cell cycle progression through effects mediated via induction of c-Myc by the Src kinase signaling pathway. GATA-1-induced differentiation is associated with repression of c-Kit and its downstream substrates Vav1, Rac1, and Akt. Enforced expression of each of these molecules individually inhibits GATA-1-regulated cell cycle arrest to different extents without affecting the induction of erythroid maturation markers. Chromatin immunoprecipitation (ChIP) studies reveal that GATA-1 occupies a positive regulatory element in the Kit gene in vivo, suggesting a direct mechanism of transcriptional repression. These results highlight a distinct antiproliferative program of GATA-1 that is related to gene repression and can be uncoupled from its ability to activate erythroid marker genes during terminal maturation. In particular, GATA-1 induces cell cycle arrest by blocking expression of multiple components of a c-Kit signaling cascade that lead to c-Myc activation. Our results provide insight into how c-Kit and GATA-1 interrelate during normal hematopoiesis and how mutations in these two essential genes might cause cytopenias and leukemias.
MATERIALS AND METHODS
Cell culture.
G1E-ER2 and G1E-ER4 are two independent clones derived from the same parental G1E cells engineered to express a conditional form of GATA-1 that is activated by estradiol or tamoxifen (GATA-1-estrogen receptor [ER] [GATA-1 fused to the ligand-binding domain of the estrogen receptor {25, 34, 61, 82}]). In the present study, similar results were obtained using both clones. The cells were grown in Iscove's modified Dulbecco's medium (InVitrogen, Rockville, MD) with 15% heat-inactivated fetal bovine serum (Bio-Whittaker, Hanover Park, IL), recombinant erythropoietin (2 U/ml; Amgen, Thousand Oaks, CA), and recombinant rat SCF (50 ng/ml; Amgen, Thousand Oaks, CA). β-Estradiol (10−7 mol/liter) was used to activate GATA-1-ER and trigger terminal erythroid maturation. (Sigma, St. Louis, MO). Src inhibitor (PP1; Biomol, Plymouth Meeting, PA), phosphatidylinositol (PI) 3-kinase inhibitor (Wortmannin; Calbiochem, San Diego, CA), and MEK inhibitor (PD98059; Calbiochem, San Diego, CA) were prepared in dimethyl sulfoxide.
Flow cytometry.
G1E-ER2 or G1E-ER4 cells were stained with an antibody against the cell surface erythroid maturation marker Ter119, as previously described (34, 61).
Microarray experiments.
In three independent experiments, G1E-ER4 cells growing in log phase were induced for 0, 3, 7, 14, 21, or 30 h with 10−7 M β-estradiol. RNA from 5 × 107 G1E-ER4 cells was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) and processed for hybridization to Affymetrix MG-U74Av2 GeneChips (23). All additional analysis was performed as previously reported (82).
Expression of c-Kit, Akt, Rac1, and Rac2.
cDNAs encoding wild-type murine c-Kit, Akt, Rac1, and Rac2 were cloned into the bicistronic retroviral vector MIEG3 (86) and verified by sequencing. The construction of wild-type and mutant chimeric c-Kit-macrophage colony-stimulating factor (M-CSF) receptors was described previously (33, 42, 70). Viral supernatants for infection of G1E-ER4 cells were generated using the Phoenix ecotropic packaging cell line transfected with retroviral vector plasmids using the Lipofectamine Plus reagent (Invitrogen, Carlsbad, CA) (33). Supernatants were collected 48 h posttransfection and filtered through 0.45-μm membranes. Cells were infected with 2 ml of high-titer virus supernatant in the presence of 8 μg/ml polybrene. Forty-eight hours after infection, enhanced green fluorescent protein (EGFP)-expressing cells were purified by fluorescence-activated cell sorting (FACS). Cells expressing similar levels of EGFP and chimeric c-Kit-M-CSF receptors were utilized in the experiments described here.
Cell cycle analysis.
G1E-ER2 or G1E-ER4 cells (5 × 106) expressing various cDNAs were grown either in the presence or in the absence of indicated inhibitors. Cell cycle distribution was determined by staining cells with propidium iodide (0.1 mg/ml containing 0.6% NP-40) in the presence of RNase A (2 mg/ml) for 30 min at 4°C, followed by flow cytometric analysis to assess DNA content.
Western blot analysis.
A total of 1 × 106 to 2 × 106 parental G1E-ER2 or G1E-ER4 cells expressing various mutants of c-Kit, Akt, and Rac were cultured for 24 and 48 h at 37°C. Thereafter, cells were harvested and lysed in cell lysis buffer (10 mM K2HPO4, 1 mM EDTA, 5 mM EGTA, 10 mM MgCl2, 1 mM Na2VO4, 50 mM β-glycerol phosphate, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 μg/ml pepstatin A containing 0.5% Triton X-100 [pH 7.2]). An equal amount of protein was fractionated on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel and electrophoretically transferred to a nitrocellulose membrane. Expression of c-Kit, Vav1, Akt, c-Myc, and CDK4 was determined by using specific antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, and Cell Signaling, Beverely, MA).
Predicting cis-regulatory modules in c-Kit by comparative genomics.
Bioinformatic predictions used whole-genome sequence alignments of mouse and human generated by blastZ (62) or mouse, rat, and human generated by multiZ (7). The regulatory potential (RP) score is a log-likelihood measure that estimates a probability that the function under selection is a cis-acting gene regulatory element (36). In this method, alignments are scored for how well they match patterns that distinguish alignments in regulatory regions from those in neutral DNA. The database of Genome Alignments and Annotations (GALA) (18) was searched to find DNA intervals in the Kit locus whose alignments had an RP score of at least 2.3, an empirically derived threshold based on identification of known regulatory elements in the Hbb locus (22). GALA was also queried for all mouse sequences in the Kit locus that match weight matrices for GATA-1 binding sites in TRANSFAC (30) and for blocks in the mouse-rat-human three-way alignments containing conserved matches to GATA-1 weight matrices (computed by tffind) (84). The DNA intervals exceeding the RP score threshold that also contain a conserved, predicted GATA-1 binding site were identified by intersection operations in GALA. Data from GALA were displayed as custom tracks in the UCSC Genome Browser to provide a consolidated view.
ChIP assays.
ChIP was performed as described previously (40, 41). Briefly, 1 × 108 cells were cross-linked in 0.4% formaldehyde-phosphate-buffered saline for 10 min at room temperature. Cross-linking was stopped with 0.125 M glycine. Chromatin was sonicated, precleared with irrelevant antibodies, and immunoprecipitated with indicated antibodies prebound to protein G-Sepharose beads. Beads were washed six times, and the bound proteins were eluted into 100 mM NaHCO3-1% SDS. Cross-links were reversed at 65°C for 4 h, and protein was digested with proteinase K (0.3 mg/ml) for 2 h at 45°C. DNA was purified by phenol-chloroform extraction and ethanol precipitation. Real-time PCR was performed and quantified using SYBR green dye on an ABI 7500 real-time PCR machine. PCR product signals were referenced to a dilution series of the relevant input and normalized to the corresponding isotype control signal for each primer pair. Primer pairs used for PCR were as follows: for Kit 1, 5′-CCTCCAGGTGCGCTATGC-3′ (forward) and 5′-TTTTACTTTATGCCTATGGGTGCTT-3′ (reverse); for Kit 2, 5′-GACAAAGGACAGAAAAACAC-3′ (forward) and 5′-GGGAGAACGGATGGGCCAGTT-3′ (reverse); for Kit 3, 5′-CACCTCCACCATAAGCCGAAT-3′ (forward) and 5′-CTCCTAGACAATAAAGGACAACCA-3′ (reverse); for Kit 4, 5′-GATTGCTGGAGGTTGTGTGA-3′ (forward) and 5′-CGCTGGAGACCACCCACTT-3′ (reverse); for Kit 5, 5′-GGCTGGAAACCACTGCCTTA-3′ (forward) and 5′-AGCCTTGCCTGTGCTTAAAGC-3′ (reverse); for Kit 6, 5′-CAGCACGCACCTCACAGAA-3′ (forward) and 5′-TCACGCAGTCTCCTTAACTCTT-3′ (reverse); for Kit 7, 5′-GGCAGGAATAAAACGGGTGTT-3′ (forward) and 5′-AAGGACTTGCTTTCCCAAACTG-3′ (reverse); for Kit 8, 5′-GGGCATTCCACAGTCATGATT-3′ (forward) and 5′-GGTCTCTCCCACCTTACTC-3′ (reverse).
RESULTS
G1E-ER4 and G1E-ER2 are two different clonal G1E cell lines engineered to stably express a conditional estrogen-activated form of GATA-1 (GATA-1-ER [GATA-1 fused to the ligand-binding domain of the estrogen receptor]). Results obtained using G1E-ER4 cells are shown below, but similar results occurred when the G1E-ER2 line was used. Consistent with our previously published results, treatment of G1E-ER4 cells with β-estradiol restored GATA-1 function, thereby inducing synchronous and homogeneous G1-phase cell cycle arrest and erythroid maturation in a fashion that largely recapitulates normal stages of erythropoiesis (Fig. 1 ) (61).
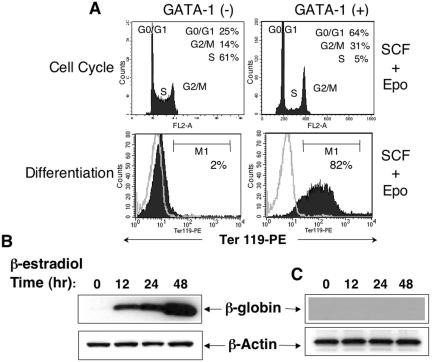
FIG. 1.
Effect of GATA-1 on cell cycle progression and terminal differentiation in G1E-ER4 cells. A total of 5 × 106 cells were cultured in SCF (50 ng/ml) and Epo (2 U/ml) in the absence (−) or presence (+) of GATA-1 expression for 48 h. Cells were harvested, and cell cycle analysis and Ter119 expression were performed by propidium iodide staining followed by FACS analysis, respectively. (A) Cell cycle analysis (top panel) and differentiation (bottom panel) in the absence or presence of β-estradiol treatment. Percentages of cells in various stages of the cell cycle are indicated. Differentiation was analyzed by monitoring the expression of Ter119 on the cell surface by flow cytometry. Open histograms indicate the level of background staining as determined by isotype control antibody. Closed histograms indicate the level of Ter119 expression. (B and C) Western blot demonstrating a time-dependent induction of globin expression in the presence (B) or in the absence (C) of β-estradiol. One of several representative experiments with similar results is shown. PE, phycoerythrin.
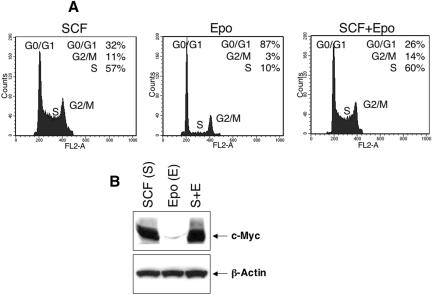
G1E cells depend on SCF and Epo for growth and survival and undergo rapid apoptosis when these cytokines are withdrawn (25, 34, 81). Epo and SCF act at successive overlapping stages of erythropoiesis, yet their individual roles in regulating erythroid cell cycle progression are not known. We examined these roles at various stages of GATA-1-induced G1E cell maturation. Consistent with our previously reported observations, G1 arrest in these cells began as early as 12 h after β-estradiol-induced GATA-1 activation; by 48 h, only 5% of the cells remained in S phase (Fig. 1A, top panels). Concurrently, these cells acquired phenotypic and morphological characteristics of terminal maturation including induction of the maturation marker Ter119 and expression of β-globin (Fig. 1A and B and data not shown). We next examined the individual contributions of SCF and Epo on cell cycle progression of erythroblasts. To investigate these roles in the absence of GATA-1, G1E-ER4 cells were cultured with either or both cytokines and cell cycle analysis was performed after 48 h. As shown in Fig. 2A (middle panel), Epo stimulation did not appreciably enhance S-phase entry. In contrast, SCF stimulation significantly increased DNA synthesis to the level observed with combined stimulation by both SCF and Epo (Fig. 2A, left and right panels). Increased S-phase entry induced by SCF was also reflected by increased expression of c-Myc, compared to Epo-stimulated cells (Fig. 2B). These results suggest that SCF, but not Epo, is the principle inducer of DNA synthesis in G1E-ER4 erythroblasts and that this might occur through induction of c-Myc.
FIG. 2.
Effect of SCF and Epo on cell cycle progression and expression of c-Myc in the absence of GATA-1 expression. A total of 5 × 106 cells were cultured in SCF (50 ng/ml), Epo (2 u/ml), or both (S+E) in the absence of GATA-1 expression for 48 h. (A) Cell cycle profiles in the presence of SCF, Epo, or both cytokines. (B) Expression of c-Myc as determined by Western blot analysis. One of three representative experiments with similar results is shown.
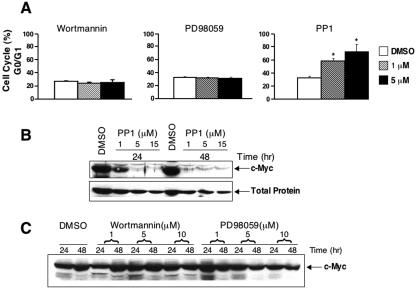
SCF binding to its receptor c-Kit stimulates several distinct signaling pathways, including members of Src family kinases, PI 3-kinase and Ras mitogen-activated protein kinase (39, 44, 64, 72, 75). To determine the role of these pathways in SCF-induced erythroblast cell cycle progression, we cultured G1E-ER4 cells in the presence of Epo plus SCF and analyzed the effects of various pharmacologic inhibitors, including PP1 (Src kinase inhibitor), wortmannin (PI 3-kinase inhibitor) and PD98059 (mitogen-activated protein kinase inhibitor) on cell cycle progression. Only the Src family kinase inhibitor PP1 induced significant G0/G1 accumulation in a dose-dependent fashion (Fig. 3A). Moreover, SCF-induced c-Myc expression was specifically blocked by PP1 (Fig. 3B). Consistent with their lack of effect on cell cycle progression, wortmannin or PD98059 did not significantly modulate the expression of c-Myc (Fig. 3A and C). These findings suggest that in erythroid cells, c-Kit stimulates cell cycle progression specifically by activating the Src kinase pathway.
FIG. 3.
Effect of PI 3-kinase, mitogen-activated protein kinase, and Src kinase pathway on SCF-induced cell cycle progression in G1E-ER4 cells. (A) G1E-ER4 cells were cultured in the presence of SCF (50 ng/ml) and wortmannin or PD98059 or PP1. Cell cycle analysis was performed by propidium iodide staining followed by FACS analysis. Bars denote the percentage of cells in G0/G1 phase of cell cycle ± standard deviation (SD) of one of the two representative experiments performed in triplicates. Asterisks indicate P < 0.05 for 1 μM and 5 μM PP1 versus dimethyl sulfoxide (DMSO). (B and C) G1E-ER4 cells were cultured in the presence of SCF (50 ng/ml) and increasing doses of PP1, wortmannin, or PD98059. An equal amount of protein was resolved on a 10% SDS-polyacrylamide gel and subjected to Western blot analysis with an anti-c-Myc antibody. Position of c-Myc is indicated. As a loading control, the same blot was probed with an anti-CDK2 antibody (total protein [B]).
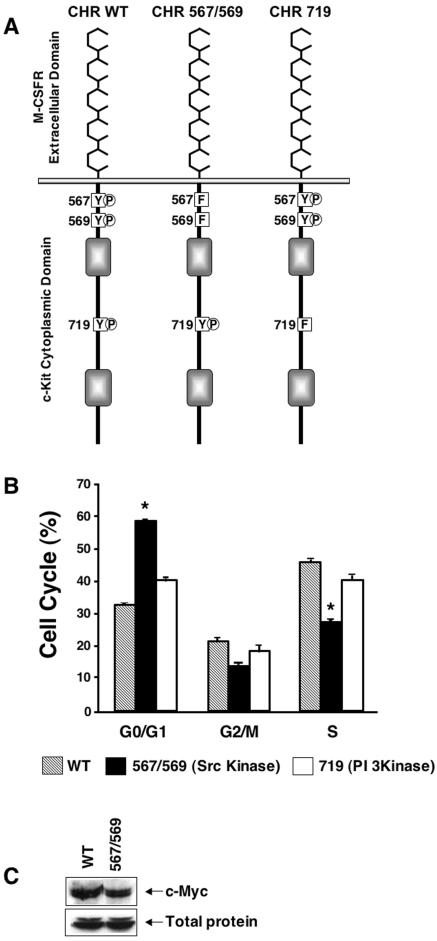
To further clarify the role of Src kinase signaling in c-Kit-induced cell cycle progression, we cloned and expressed c-Kit mutant receptors impaired in their ability to activate the Src and PI 3-kinase pathways. Since G1E-ER2 cells express endogenous c-Kit receptors, we constructed a chimeric c-Kit receptor (CHR) to bypass endogenous c-Kit receptors and study the effects of specific signaling mutants (Fig. 4A). The M-CSF receptor and c-Kit belong to the same subfamily of RTKs but possess distinct ligand binding specificities (76). G1E-ER2 cells do not express endogenous M-CSF receptor and show no response to M-CSF stimulation (70). This CHR is activated upon binding M-CSF but signals in a fashion similar to that of the endogenous c-Kit receptor (33, 70). Using this receptor as a template, c-Kit receptors with mutant intracellular signaling domains were generated by replacing tyrosine for phenylalanine at the binding sites for Src family kinases (position 567/569) or PI 3-kinase (position 719), which abrogates the respective signaling pathways (33, 39, 42, 44, 64, 70-72, 75). We then generated G1E-ER2 lines that stably express wild-type and mutant CHR receptors and examined their activities by culturing the cells in M-CSF, without SCF. c-Kit mutations that abrogate Src kinase binding and activation were deficient in promoting G1-to-S-phase cell cycle progression and also failed to upregulate c-Myc normally (Fig. 4B and C). In contrast, inhibiting the PI 3-kinase pathway had no effects on cell cycle progression (Fig. 4B) or c-Myc induction (data not shown). Collectively, these results suggest an important and specific role for c-Kit-mediated activation of the Src family kinase in regulation of c-Myc expression and erythroblast cell cycle progression. Consistent with previous findings in other cell types, it is likely that Src kinase activation stimulates erythroblast proliferation at least in part by inducing c-Myc (9, 15).
FIG. 4.
Inhibition of cell cycle progression and expression of c-Myc by mutating the binding sites for Src family kinases in c-Kit. (A) Schematic structure of wild-type (WT) and mutant CHRs. The c-Kit CHR includes the ligand-binding domain of the human M-CSF receptor, the transmembrane, and the cytoplasmic tail of the c-Kit receptor. In the mutant c-Kit CHRs, tyrosines (Y) at the indicated positions have been changed to phenylalanine (F). (B) G1E-ER2 cells expressing wild-type CHR, CHR mutated at position 567/569 (CHR 567/569), or CHR 719 were cultured in the presence of M-CSF (200 ng/ml). After 48 h, cells were harvested, and cell cycle analysis was performed by propidium iodide staining followed by FACS analysis. Bars indicate the percentage of cells in G0/G1, G2/M, and S phases of the cell cycle ± SD of one of two representative experiments performed in triplicates. Asterisks indicate P < 0.05 for CHR 567/569 versus wild-type CHR or CHR 719. (C) G1E-ER2 cells expressing the indicated receptor (wild-type CHR or CHR 567/569) were cultured in M-CSF (200 ng/ml). Cells were collected and lysed after 24 h, and an equal amount of protein was subjected to Western blot analysis with an anti-c-Myc antibody. One of three experiments with similar results is shown.
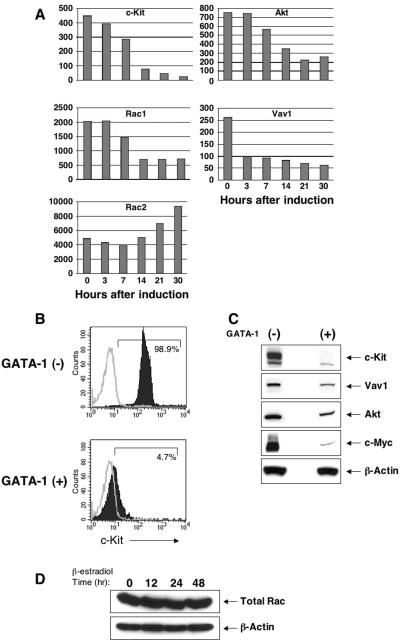
Previously, we demonstrated that downregulation of c-Myc is required for GATA-1-induced cell cycle arrest in G1E-ER4 cells and that GATA-1 binds the Myc gene coincident with its repression (also see Fig. 5C) (61). These findings indicated that GATA-1 inhibits erythroblast proliferation by directly inhibiting c-Myc transcription. However, given the current findings that c-Myc expression in uninduced G1E-ER4 cells is regulated largely via c-Kit-activated Src kinase (Fig. 2 and 3), we considered the possibility that GATA-1-mediated repression of genes involved in this signaling pathway could also contribute to inhibition of c-Myc expression during terminal erythropoiesis. Recently, we used oligonucleotide microarrays to define the kinetics of GATA-1-induced changes in gene expression in G1E-ER4 cells (82). We interrogated these data for the expression of c-Kit and its Src-related downstream signaling effectors (Fig. 5A). Of note, mRNAs encoding c-Kit and the intracellular kinases Vav1, Akt, and Rac1 are all rapidly downregulated during GATA-1-induced G1E cell maturation (Fig. 5A). Flow cytometry and Western blot studies showed that c-kit, Vav1, and Akt proteins were also downregulated after GATA-1 activation (Fig. 5B and C). Total Rac protein remained constant (Fig. 5D), probably because coincident with Rac1 repression, the related protein Rac2 was reciprocally upregulated (Fig. 5A). Previous studies in other cell systems showed that Akt promotes the expression of c-Myc and that the Src kinase/Vav/Rac pathway plays an essential role in regulating the expression of c-Myc in response to platelet-derived growth factor (PDGF) stimulation in fibroblasts (9, 15, 58, 63). c-Kit activates Akt, Vav, Rac1, and Rac2, and deficiency of some of these molecules reduces SCF-induced proliferation and/or survival (1, 26, 27, 33, 72, 86). Hence, during erythroid maturation, GATA-1-mediated repression of c-Kit and multiple downstream effectors that signal through the Src kinase pathway may contribute to c-Myc repression and consequent proliferation arrest. In particular, c-Kit, Vav1, Rac1, and Akt are all repressed (directly or indirectly) by GATA-1. Upregulation of Rac2 during the same time frame may indicate unique c-Kit-independent roles for this Rho GTPase in erythroid maturation.
FIG. 5.
Conditional activation of GATA-1 in G1E-ER4 cells results in repression of c-Kit and its downstream substrates. (A) Time-dependent decline in the mRNA levels of c-Kit, Vav1, Rac1, and Akt in the presence of GATA-1 activation. The y axes of the graphs indicate the absolute signal value from microarrays. Hours after β-estradiol treatment are indicated. Similar results were found in three independent experiments. (B) Cell surface expression of c-Kit in the absence (−) and presence (+) of GATA-1. G1E-ER4 cells were cultured for 48 h in the presence of β-estradiol and subjected to flow cytometric analysis using an anti-c-Kit antibody. Open histograms indicate isotype control staining, and closed histograms indicate c-Kit staining. (C and D) Western blot analysis on lysates derived from G1E-ER4 cells cultured in the absence or in the presence of GATA-1 activation for 48 h. Similar results were observed in three independent experiments.
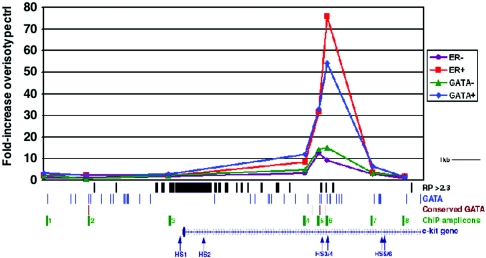
GATA-1 could inhibit the expression of c-Kit signaling components either directly or indirectly. We investigated this issue by first focusing on the c-Kit receptor itself, which is rapidly repressed following GATA-1 activation in G1E-ER4 cells (Fig. 5). Recent studies identified a segment of the Kit gene promoter and first intron that drives expression of a linked green fluorescence protein reporter in hematopoietic cells of transgenic mice (13). This region contains six DNase hypersensitive (HS) sites that are implicated in control of gene expression (Fig. 6). We examined these sites for in vivo GATA-1 occupancy by ChIP (Fig. 6). An upstream region of the Kit promoter without any conserved GATA sites was selected as a negative control (ChIP amplicon 1 in Fig. 6). ChIP was performed in G1E-ER4 cells before and 24 h after β-estradiol-induced activation of GATA-1. Occupancy of the GATA-1-ER fusion protein was measured using two different antibodies, one directed against GATA-1 and one directed against the ER moiety. We found prominent GATA-1 binding to the region of DNase HS 3/4 in the first intron of the Kit gene (ChIP amplicons 5 and 6 in Fig. 6). This region contains three sequence elements that are predicted to be involved in transcriptional regulation using bioinformatic approaches (18) (“RP > 2.3” track in Fig. 6) and two GATA-binding sites that are conserved in evolution (“conserved GATA” track in Fig. 6). Hence, GATA-1 binds a regulatory element in the c-Kit gene coincident with its downregulation, suggesting a direct mechanism for gene repression. Of note, we found no GATA-1 binding to the c-Kit promoter where GATA-1 was previously implicated to act in regulating gene expression (38). However, GATA-1 is believed to bind this region of the c-Kit gene indirectly, which is predicted to lower the sensitivity of the ChIP assay.
FIG. 6.
Quantitative ChIP analysis of GATA-1-ER binding at the Kit locus before (−) and after (+) β-estradiol treatment of G1E-ER4 cells for 24 h. The 5′ end of the Kit gene encompassing a region previously shown to recapitulate expression of the endogenous locus in transgenic mice is diagrammed at the bottom, with exon 1 represented by a taller box. The direction of transcription is from left to right, as indicated by arrows. DNase HS sites reported to mark functionally important regions of the locus (13) are indicated as vertical arrows. Matches to weight matrices for GATA-1 binding sites were identified in the mouse sequence (track labeled “GATA”), and these were filtered to find sites that are conserved in mouse, rat, and human alignments (track labeled “conserved GATA”). RP (top line) refers to an arbitrary score generated by a computer algorithm used to predict gene regulatory regions (18); regions with scores above 2.3, which is considered to represent a stringent threshold for significance, are shown as black vertical bars. Chromatin from G1E-ER4 cells was purified before (−) and 24 h after (+) estradiol treatment, immunoprecipitated with antibodies against the GATA-1 (GATA) or ER portions of the GATA-1-ER fusion protein, and analyzed for Kit gene sequences represented by the ChIP amplicons 1 to 8, shown in green. The corresponding signals are plotted graphically at the top of the figure. Each data point represents the average value obtained from at least two independent experiments normalized to the corresponding isotype control signal for the ChIP amplicon PCR primer pair. The information in these tracks was obtained by queries to GALA, and the data were visualized in the UCSC Genome Browser.
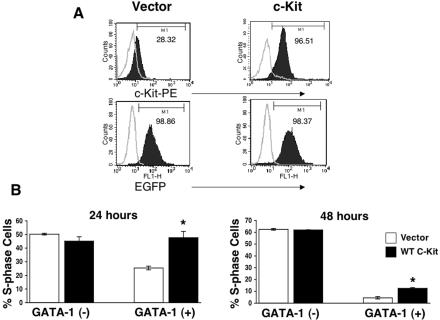
If GATA-1 inhibits G1E-ER4 proliferation by binding the Kit gene and repressing its expression, then enforced expression of c-Kit in G1E-ER4 cells should override GATA-1-induced cell cycle arrest and c-Myc repression. To test this, we engineered G1E-ER4 cells to express constitutive c-Kit using a bicistronic retroviral vector containing EGFP to track transduced cells. c-Kit-EGFP-expressing G1E-ER4 cells were sorted to homogeneity and then treated with β-estradiol to activate GATA-1. As seen in Fig. 7A (left panel), activation of GATA-1 in G1E-ER4 cells transduced with empty vector led to the loss of surface c-Kit protein with no effect on EGFP expression after 48 h of β-estradiol treatment. In contrast, surface c-Kit expression was sustained after GATA-1 activation in c-Kit virus-transduced cells (Fig. 7A, right panel). Consistent with our hypothesis, enforced expression of c-Kit specifically inhibited GATA-1-induced withdrawal of cells from S phase (Fig. 7B). This effect was significant throughout terminal maturation but more pronounced at 24 h than at 48 h. Hence, persistent c-Kit expression partially abrogates GATA-1-mediated cell cycle arrest, suggesting that c-Kit repression is important for cell cycle withdrawal during erythroid maturation. Importantly, these findings contrast with our recent studies demonstrating that enforced c-Myc expression induces a more complete reversal of GATA-1-mediated G1E-ER4 cell cycle arrest (61), even at later time points. We believe that in β-estradiol-treated G1E-ER4 cells, the mitogenic effects of constitutive c-Kit expression are attenuated by GATA-1-mediated repression of key downstream signaling molecules including Vav1, Rac1, and Akt and c-Myc itself (Fig. 5 and 6) (1, 33, 72).
FIG. 7.
Sustained expression of c-Kit in G1E-ER4 cells results in partial reversal of GATA-1-induced S-phase repression. (A) Cell surface expression of c-Kit in G1E-ER4 cells transduced with c-Kit induced to express GATA-1 for 48 h. Upper left panel, GATA-1-expressing G1E-ER4 cells transduced with the empty vector; upper right panel, GATA-1-expressing G1E-ER4 cells transduced with c-Kit. The bottom panel indicates EGFP expression in both lines. In upper panels (left and right), open histograms indicate the level of background expression as measured by isotype control staining. Closed histograms indicate the level of c-Kit expression. In the bottom panels, closed histograms indicate the level of EGFP expression in transduced cells compared to that of nontransduced cells (open histograms). (B) G1E-ER4 cells overexpressing c-Kit or the empty vector were subjected to cell cycle analysis in the absence or in the presence of GATA-1 expression after 24 and 48 h. Bars denote the percentage of cells in S phase of the cell cycle ± SD of two independent experiments performed in triplicates. Asterisk indicates P < 0.05 (c-Kit versus vector).
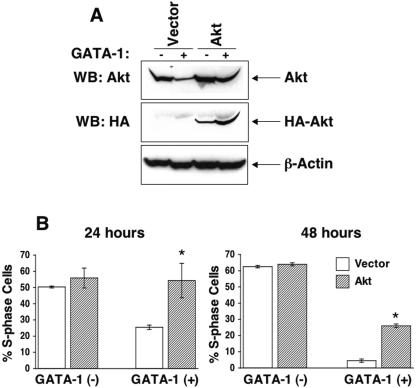
To determine the relative contribution of individual Src kinase pathway signaling molecules in GATA-1-induced cell cycle repression via c-Kit, we cloned the cDNAs encoding wild-type forms of murine Akt, Rac1, and Rac2 into a bicistronic retroviral vector and expressed them in G1E-ER4 cells. EGFP-expressing cells were sorted to homogeneity and analyzed for cell cycle and proliferation status after GATA-1 activation. Figure 8A demonstrates sustained expression of Akt in G1E-ER4 cells expressing GATA-1. Sustained expression of Akt in G1E-ER4 cells resulted in significantly higher level of cells in the S phase of the cell cycle compared to those expressing either the empty vector or c-Kit (Fig. 8B).
FIG. 8.
Sustained expression of Akt in G1E-ER4 cells results in partial reversal of GATA-1-induced S-phase repression. (A) Akt expression in G1E-ER4 cells transduced with either the empty vector or a cDNA encoding wild-type hemagglutinin (HA)-tagged Akt. WB, Western blot. (B) G1E-ER4 cells overexpressing Akt or the empty vector were subjected to cell cycle analysis in the absence or in the presence of GATA-1 expression after 24 and 48 h. Bars denote the percentage of cells in S phase of the cell cycle ± SD of two independent experiments performed in triplicates. The asterisks indicate P < 0.05 (Akt versus vector).
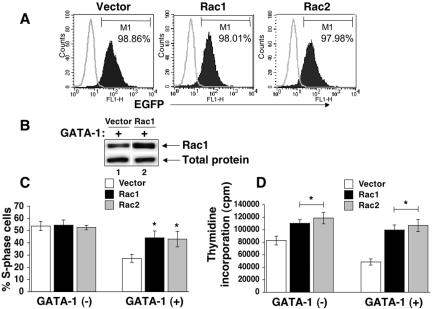
Previous studies implicate a critical role for Rho GTPases in regulating cell cycle progression in other cell systems as well as in response to c-Kit (33, 72). Furthermore, deficiency of Rac1 but not Rac2 in c-Kit+ hematopoietic stem/progenitor cells results in cell cycle defects (15, 27). Since induction of GATA-1 in G1E-ER4 cells represses Rac1 mRNA, we used retroviral transduction to examine the consequences of sustained expression of Rac GTPases on GATA-1-induced cell cycle arrest (Fig. 9). Overexpression of either Rac1 or Rac2 significantly inhibited GATA-1-induced proliferation arrest in G1E-ER4 cells, as measured by the fraction of S-phase cells (Fig. 9C) and DNA replication rates (Fig. 9D). This suggests that Rac1 and Rac2 function similarly in promoting erythroid cell cycle progression. The reciprocal regulation of these related proteins during G1E-ER4 cell maturation (Fig. 5A and see above) might reflect distinct functions for Rac2 that are required during terminal maturation (see Discussion).
FIG. 9.
Sustained expression of Rac1 or Rac2 in G1E-ER4 cells results in reversal of GATA-1-induced S-phase repression. (A) Flow cytometric analysis of Rac1 and Rac1 expression as determined by EGFP staining. Open histograms indicate background staining in non-EGFP-expressing G1E-ER4 cells. Closed histograms indicate Rac1 or Rac2 expression as reflected by the expression of EGFP. (B) Western blot analysis of Rac1 expression in G1E-ER4 cells transduced with a retrovirus encoding Rac1 cDNA. G1E-ER4 cells overexpressing Rac1 or Rac2 or an empty vector were subjected to cell cycle analysis (C) or a thymidine incorporation assay (D) in the absence or in the presence of GATA-1 expression after 24 h. Bars denote the thymidine incorporation or the percentage of cells in S phase of the cell cycle ± SD of two independent experiments performed in triplicates. Asterisks indicate P < 0.05 (Rac1 or Rac2 versus vector).
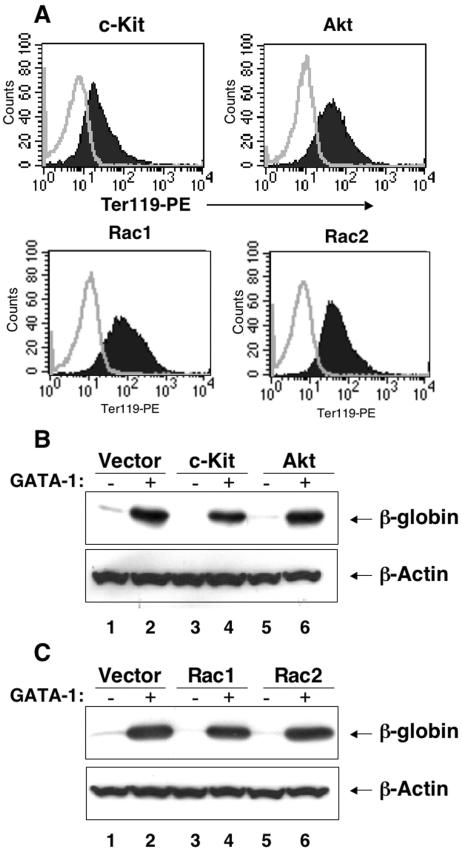
Surprisingly, while enforced c-Kit, Akt, Rac1, and Rac2 expression impaired GATA-1-mediated cell cycle withdrawal in G1E-ER4 cells, other GATA-1 functions related to terminal maturation were relatively undisturbed. Upon treatment with β-estradiol for 24 or 48 h, c-Kit-, Akt-, Rac1-, or Rac2-overexpressing G1E-ER4 cells induced Ter119 and β-globin expression relatively normally (Fig. 10 and data not shown). Importantly, induction of Ter119 at early time points (24 h), when most of the c-Kit-, Akt-, Rac1-, or Rac2-overexpressing G1E-ER4 cells were in cycle, was similar to vector-only-expressing cells (data not shown). These results illustrate that cell cycle arrest was uncoupled from differentiation in these cells. Together, these findings indicate that repression of c-Kit and its downstream substrates is required for G1E-ER4 GATA-1 actions related to cell cycle arrest. In contrast, functions of GATA-1 related to acquisition of the mature erythroid phenotype are largely c-Kit, Rac1, Rac1, or Akt independent.
FIG. 10.
Sustained expression of c-Kit, Akt, Rac1, or Rac2 in G1E-ER4 cells does not affect differentiation. (A) G1E-ER4 cells expressing the above-mentioned cDNAs were induced to differentiate in the presence of β-estradiol for 48 h and subjected to flow cytometric analysis to detect the expression of Ter119. Closed histograms indicate staining using an isotype control antibody. Open histograms indicate staining using an antibody against Ter119. (B and C) Cell lysates from panel A were subjected to Western blot analysis using an anti-globin antibody.
DISCUSSION
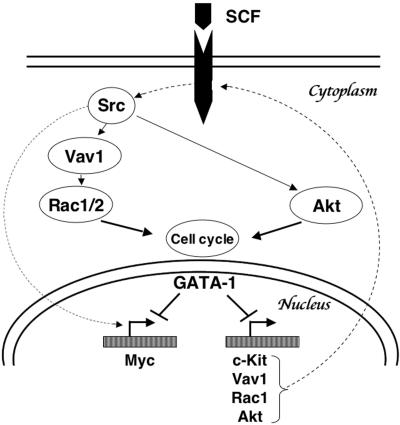
Normal blood cell differentiation is accompanied by an increasingly restricted proliferative capacity that usually culminates in G1 arrest. In the erythroid lineage, c-Kit and GATA-1 play essential roles in regulating expansion and maturation of erythroblasts, respectively. Downregulation of c-Kit signaling is essential for normal differentiation, as SCF retards the differentiation of erythroblasts while stimulating proliferation (53). Moreover, gain-of-function mutations of Kit in humans are associated with myeloproliferative diseases, including acute myelogenous leukemia and mastocytosis. Likewise, germ line GATA-1 mutations in humans are associated with leukemia (28). While these findings support a role for GATA-1 in restraining the proliferation of hematopoietic precursors, the associated mechanisms are poorly understood. Furthermore, the functional link between c-Kit and GATA-1 in erythroid cells is unclear. Here, we demonstrate that c-Kit is a major contributor to cell cycle progression and that GATA-1 represses c-Kit signaling at multiple levels during erythroid differentiation. Enforced expression of c-Kit or its downstream effectors reverses GATA-1-mediated effects on cell cycle arrest but not differentiation. The current study, together with our previous findings that GATA-1 represses c-Myc expression directly (61), defines a regulated signaling cascade, in which GATA-1 inhibits the expression of c-Kit, Rac1, Akt, and c-Myc (directly or indirectly), to induce G1 arrest during terminal maturation (Fig. 11).
FIG. 11.
Model outlining the mechanism(s) of cell cycle progression and arrest in erythroblasts involving GATA-1 and components of the c-Kit signaling cascade.
Our findings are consistent with recent studies demonstrating reversal of tumorigenicity and differentiation in an erythroleukemic cell line by enforced expression of GATA-1 (16). MEL cells are malignant erythroblasts that proliferate continuously and are blocked from differentiating into mature erythroid cells (47). Enforced expression of GATA-1 in MEL cells induces terminal erythroid differentiation in vitro and reverses tumorigenicity in vivo (16). Of note, terminal differentiation and reduced proliferation in these cells were associated with repression of c-Kit and c-Myc (16, 32).
The antiproliferative effects of GATA-1 demonstrated here provide a biochemical and mechanistic basis for some of the erythropoietic defects observed in GATA-1-deficient mice (60, 79). While complete loss of GATA-1 causes proerythroblast apoptosis, hypomorphic alleles can lead to excessive proliferation (69, 77, 80). This may be because low levels of GATA-1 are sufficient to rescue apoptosis, while higher physiologic levels are required for antiproliferative activities. For example, heterozygous female mice carrying a mutation in the GATA-1 promoter that reduces expression to 5% of normal levels accumulate erythroblasts in the spleen and develop pancytopenia and leukemia (65, 69). Likewise, another mouse mutant carrying a GATA-1 promoter mutation, which expresses about 10 to 20% of normal GATA-1 levels in erythroblasts, also develops erythroid hyperplasia (78). It is likely that the pathological erythroblast proliferation observed in these animals derives, at least in part, from insufficient GATA-1 to suppress the c-Kit-Src-Myc signaling axis described here. In this regard, the ability of GATA-1 to inhibit this signaling pathway at multiple levels highlights a broad-based and likely redundant mechanism to enforce cell cycle control. This probably explains why sustained expression of c-Kit or its downstream effectors Rac1, Rac2, and Akt individually was not sufficient to completely reverse the effects of GATA-1 activation on cell cycle arrest, especially at later time points, when multiple signaling components are repressed. Likewise, enforced expression of c-Kit, Akt, or Rac alone did not override GATA-1-induced repression of c-Myc (data not shown). Hence, these repressive effects of GATA-1 interact cooperatively and exhibit smaller influences when analyzed individually. In vivo, it is likely that this cell cycle control mechanism is dynamically regulated and subject to temporal, environmental, and developmental influences. For example GATA-1 is gradually induced during normal erythropoiesis, and it is possible that different levels are required for repression of various individual components of the c-Kit signaling pathway and c-Myc. It is also possible that there are additional mechanisms of GATA-1-mediated cell cycle arrest that are independent of c-Kit and c-Myc repression (61). To this end, we have evidence to suggest that sustained expression of c-Kit or Akt in GATA-1-expressing erythroblasts promotes expression of CDK2 and blocks induction of p27 by GATA-1 (data not shown).
Our data indicating repression of Rac1 and reciprocal induction of Rac2 by GATA-1 are intriguing and require further investigation. Although the functions of Rac GTPase proteins in regulating actin assembly and motility in fibroblasts are well documented (29), their role in blood cell development is only recently being elucidated. Rac2 is expressed specifically in hematopoietic cells, whereas Rac1 is ubiquitous (50, 66). Deficiency of Rac1 and Rac2 in hematopoietic cells results in both overlapping and unique defects (27). For example, deficiency of Rac1 but not Rac2 alters cell cycle progression in c-Kit+ hematopoietic progenitors (27). Deficiencies of either Rac isoform equally affect adhesion and migration of c-Kit+ cells (27). In contrast, deficiency of Rac2 but not Rac1 in neutrophils results in defective migration and superoxide production (27). These results suggest that Rac1 and Rac2 have both overlapping and distinct functions in hematopoietic cells. Mature erythrocytes lacking both of these proteins are unstable and exhibit shape abnormalities indicative of cytoskeletal defects (33a). Our current results indicate that Rac GTPase signaling might also participate in erythroblast cell cycle progression. In this regard, repression of Rac1 during terminal maturation may inhibit c-Kit-mediated proliferative signals. In contrast, upregulation of Rac2 during terminal maturation may reflect ongoing requirements related to specialized erythroid functions, such as cytoskeletal organization. Previous studies showed GATA-4 to function as a transcriptional effector of Rho GTPases in cardiomyocytes and regulate sarcomere assembly (14). On this basis, it is possible that Rac2 modulates the activity of erythroid GATA. It is also possible that Rac2 modulates signaling via the Epo or insulin-like growth factor 1 receptors, which are active during later stages of maturation (2).
The binding of GATA-1 to a defined regulatory region in the Kit gene coincident with downregulation of its mRNA strongly indicates a direct mechanism for inhibiting transcription and further highlights a generally important role for GATA-1 in gene repression. Other recently appreciated direct targets for GATA-1 repression include GATA-2 (24) and c-Myc (61). GATA-2 participates in the maintenance of hematopoietic stem cells through unknown mechanisms, while c-Myc and c-Kit are protooncogenes that drive cell proliferation. Hence, GATA-1 appears to be involved in two distinct but interrelated transcriptional programs in erythropoiesis. First, it drives terminal maturation by positively regulating many erythroid-specific genes involved in mature red blood cell function. Simultaneously, it represses the transcription of genes associated with cell division and maintenance of the immature state. Whether GATA-1 represses Vav, Akt, or Rac1 directly during erythroid maturation remains an open question.
Previous studies in fibroblasts suggest an important role for the Ras/Raf/Mek/Erk pathway in regulating cell proliferation by inducing c-Myc (35, 63). This does not appear to be the case for G1E-ER4 cells, because inhibiting this pathway either biochemically or through targeted mutations of c-Kit did not affect SCF-induced cell cycle progression. Our results demonstrate that the Src kinase pathway is more important for regulating cell cycle progression via c-Kit in erythroblasts. Utilizing pharmacologic approaches and genetic manipulation of the c-Kit receptor, we demonstrated an essential role for the Src kinase pathway in regulating cell cycle progression and expression of c-Myc in response to SCF stimulation of erythroblasts. Our findings are consistent with previous studies demonstrating that neutralizing antibodies to Src kinases and dominant negative versions of c-Src and Fyn inhibit DNA synthesis in response to PDGF receptor stimulation (10, 73). Interestingly, expression of c-Myc is sufficient to overcome c-SRC requirements for induction of DNA syntheses by RTKs (4, 6), indicating that Src kinases regulate a pathway controlling c-Myc expression. Recently, Chiariello et al. defined the mechanism of PDGF receptor-mediated c-Myc induction (15). Utilizing various dominant negative and activating mutants of Src, Vav, and Rac, they demonstrated a role for this pathway in regulating c-Myc expression in response to PDGF stimulation (15). Since PDGF receptor and c-Kit belong to the same family of RTKs and are believed to utilize similar biochemical pathways for cell cycle progression, it is not surprising that c-Kit also utilizes the Src/c-Myc axis to control cell cycle progression in erythroblasts. c-Kit stimulation by SCF results in the activation of several distinct Src family members including Lyn and Fyn (33, 72). G1E-ER4 cells express Lyn, Fgr, Fyn, and Hck (data not shown). Whether these three or additional four family members, including Fgr and Hck, contribute to c-Kit-induced cell cycle progression in erythroblasts remains an open question and is currently being analyzed using knockouts of various Src family kinases.
In summary, our results support a regulatory hierarchy in which GATA-1 inhibits cell cycle progression in differentiating erythroblasts by repressing the expression of multiple components of an SCF signaling pathway involving c-Kit, Vav, Rac-1, Akt, and c-Myc (Fig. 11). At least two of the corresponding genes, Kit and Myc, appear to be inhibited by GATA-1 directly (this paper; 61), suggesting that transcriptional repression by GATA-1 may be a general mechanism that underlies its antiproliferative activities. Moreover, enforced expression of several proteins in this pathway inhibits GATA-1-regulated cell cycle withdrawal with minimal effects on the induction of erythroid markers or morphological changes associated with terminal maturation. This indicates that GATA-1 regulates cell cycle and erythroid maturation through distinct genetic programs that can be uncoupled through genetic manipulation.
Acknowledgments
We thank Ross Hardison for his help with bioinformatic analysis. We also thank Arliene Brilt for help with manuscript preparation.
This work was supported by NIH NHLBI grant R01 HL075816 (R.K.) and NIH NIDDK grant R01 DK064037 (M.J.W.).
REFERENCES
- 1.Alai, M., A. L. Mui, R. L. Cutler, X. R. Bustelo, M. Barbacid, and G. Krystal. 1992. Steel factor stimulates the tyrosine phosphorylation of the proto-oncogene product, p95vav, in human hemopoietic cells. J. Biol. Chem. 267:18021-18025. [PubMed] [Google Scholar]
- 2.Arai, A., E. Kanda, and O. Miura. 2002. Rac is activated by erythropoietin or interleukin-3 and is involved in activation of the Erk signaling pathway. Oncogene 21:2641-2651. [DOI] [PubMed] [Google Scholar]
- 3.Bao, H., S. M. Jacobs-Helber, A. E. Lawson, K. Penta, A. Wickrema, and S. T. Sawyer. 1999. Protein kinase B (c-Akt), phosphatidylinositol 3-kinase, and STAT5 are activated by erythropoietin (EPO) in HCD57 erythroid cells but are constitutively active in an EPO-independent, apoptosis-resistant subclone (HCD57-SREI cells). Blood 93:3757-3773. [PubMed] [Google Scholar]
- 4.Barone, M. V., and S. A. Courtneidge. 1995. Myc but not Fos rescue of PDGF signalling block caused by kinase-inactive Src. Nature 378:509-512. [DOI] [PubMed] [Google Scholar]
- 5.Beghini, A., P. Peterlongo, C. B. Ripamonti, L. Larizza, R. Cairoli, E. Morra, and C. Mecucci. 2000. C-kit mutations in core binding factor leukemias. Blood 95:726-727. [PubMed] [Google Scholar]
- 6.Blake, R. A., M. A. Broome, X. Liu, J. Wu, M. Gishizky, L. Sun, and S. A. Courtneidge. 2000. SU6656, a selective Src family kinase inhibitor, used to probe growth factor signaling. Mol. Cell. Biol. 20:9018-9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanchette, M., W. J. Kent, C. Riemer, L. Elnitski, A. F. Smit, K. M. Roskin, R. Baertsch, K. Rosenbloom, H. Clawson, E. D. Green, D. Haussler, and W. Miller. 2004. Aligning multiple genomic sequences with the threaded blockset aligner. Genome Res. 14:708-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boissan, M., F. Feger, J. J. Guillosson, and M. Arock. 2000. c-Kit and c-kit mutations in mastocytosis and other hematological diseases. J. Leukoc. Biol. 67:135-148. [DOI] [PubMed] [Google Scholar]
- 9.Bowman, T., M. A. Broome, D. Sinibaldi, W. Wharton, W. J. Pledger, J. M. Sedivy, R. Irby, T. Yeatman, S. A. Courtneidge, and R. Jove. 2001. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc. Natl. Acad. Sci. USA 98:7319-7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broome, M. A., and T. Hunter. 1996. Requirement for c-Src catalytic activity and the SH3 domain in platelet-derived growth factor BB and epidermal growth factor mitogenic signaling. J. Biol. Chem. 271:16798-16806. [DOI] [PubMed] [Google Scholar]
- 11.Broudy, V. C. 1997. Stem cell factor and hematopoiesis. Blood 90:1345-1364. [PubMed] [Google Scholar]
- 12.Buttner, C., B. M. Henz, P. Welker, N. T. Sepp, and J. Grabbe. 1998. Identification of activating c-kit mutations in adult-, but not in childhood-onset indolent mastocytosis: a possible explanation for divergent clinical behavior. J. Investig. Dermatol. 111:1227-1231. [DOI] [PubMed] [Google Scholar]
- 13.Cairns, L. A., E. Moroni, E. Levantini, A. Giorgetti, F. G. Klinger, S. Ronzoni, L. Tatangelo, C. Tiveron, M. De Felici, S. Dolci, M. C. Magli, B. Giglioni, and S. Ottolenghi. 2003. Kit regulatory elements required for expression in developing hematopoietic and germ cell lineages. Blood 102:3954-3962. [DOI] [PubMed] [Google Scholar]
- 14.Charron, F., G. Tsimiklis, M. Arcand, L. Robitaille, Q. Liang, J. D. Molkentin, S. Meloche, and M. Nemer. 2001. Tissue-specific GATA factors are transcriptional effectors of the small GTPase RhoA. Genes Dev. 15:2702-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiariello, M., M. J. Marinissen, and J. S. Gutkind. 2001. Regulation of c-myc expression by PDGF through Rho GTPases. Nat. Cell Biol. 3:580-586. [DOI] [PubMed] [Google Scholar]
- 16.Choe, K. S., F. Radparvar, I. Matushansky, N. Rekhtman, X. Han, and A. I. Skoultchi. 2003. Reversal of tumorigenicity and the block to differentiation in erythroleukemia cells by GATA-1. Cancer Res. 63:6363-6369. [PubMed] [Google Scholar]
- 17.Dolznig, H., B. Habermann, K. Stangl, E. M. Deiner, R. Moriggl, H. Beug, and E. W. Mullner. 2002. Apoptosis protection by the Epo target Bcl-X(L) allows factor-independent differentiation of primary erythroblasts. Curr. Biol. 12:1076-1085. [DOI] [PubMed] [Google Scholar]
- 18.Elnitski, L., R. C. Hardison, J. Li, S. Yang, D. Kolbe, P. Eswara, M. J. O'Connor, S. Schwartz, W. Miller, and F. Chiaromonte. 2003. Distinguishing regulatory DNA from neutral sites. Genome Res. 13:64-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujiwara, Y., C. P. Browne, K. Cunniff, S. C. Goff, and S. H. Orkin. 1996. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc. Natl. Acad. Sci. USA 93:12355-12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galli, S. J., K. M. Zsebo, and E. N. Geissler. 1994. The kit ligand, stem cell factor. Adv. Immunol. 55:1-96. [DOI] [PubMed] [Google Scholar]
- 21.Gari, M., A. Goodeve, G. Wilson, P. Winship, S. Langabeer, D. Linch, E. Vandenberghe, I. Peake, and J. Reilly. 1999. c-kit proto-oncogene exon 8 in-frame deletion plus insertion mutations in acute myeloid leukaemia. Br. J. Haematol. 105:894-900. [DOI] [PubMed] [Google Scholar]
- 22.Giardine, B., L. Elnitski, C. Riemer, I. Makalowska, S. Schwartz, W. Miller, and R. C. Hardison. 2003. GALA, a database for genomic sequence alignments and annotations. Genome Res. 13:732-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golub, T. R., D. K. Slonim, P. Tamayo, C. Huard, M. Gaasenbeek, J. P. Mesirov, H. Coller, M. L. Loh, J. R. Downing, M. A. Caligiuri, C. D. Bloomfield, and E. S. Lander. 1999. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science 286:531-537. [DOI] [PubMed] [Google Scholar]
- 24.Grass, J. A., M. E. Boyer, S. Pal, J. Wu, M. J. Weiss, and E. H. Bresnick. 2003. GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling. Proc. Natl. Acad. Sci. USA 100:8811-8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregory, T., C. Yu, A. Ma, S. H. Orkin, G. A. Blobel, and M. J. Weiss. 1999. GATA-1 and erythropoietin cooperate to promote erythroid cell survival by regulating bcl-xL expression. Blood 94:87-96. [PubMed] [Google Scholar]
- 26.Gu, Y., M. C. Byrne, N. C. Paranavitana, B. Aronow, J. E. Siefring, M. D'Souza, H. F. Horton, L. A. Quilliam, and D. A. Williams. 2002. Rac2, a hematopoiesis-specific Rho GTPase, specifically regulates mast cell protease gene expression in bone marrow-derived mast cells. Mol. Cell. Biol. 22:7645-7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu, Y., M. D. Filippi, J. A. Cancelas, J. E. Siefring, E. P. Williams, A. C. Jasti, C. E. Harris, A. W. Lee, R. Prabhakar, S. J. Atkinson, D. J. Kwiatkowski, and D. A. Williams. 2003. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science 302:445-449. [DOI] [PubMed] [Google Scholar]
- 28.Gurbuxani, S., P. Vyas, and J. D. Crispino. 2004. Recent insights into the mechanisms of myeloid leukemogenesis in Down syndrome. Blood 103:399-406. [DOI] [PubMed] [Google Scholar]
- 29.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 30.Hardison, R. C., F. Chiaromonte, D. Kolbe, H. Wang, H. Petrykowska, L. Elnitski, S. Yang, B. Giardine, Y. Zhang, C. Riemer, S. Schwartz, D. Haussler, K. M. Roskin, R. J. Weber, M. Diekhans, W. J. Kent, M. J. Weiss, J. Welch, and W. Miller. 2003. Global predictions and tests of erythroid regulatory regions. Cold Spring Harb. Symp. Quant. Biol. 68:335-344. [DOI] [PubMed] [Google Scholar]
- 31.Harigae, H., S. Takahashi, N. Suwabe, H. Ohtsu, L. Gu, Z. Yang, F. Y. Tsai, Y. Kitamura, J. D. Engel, and M. Yamamoto. 1998. Differential roles of GATA-1 and GATA-2 in growth and differentiation of mast cells. Genes Cells 3:39-50. [DOI] [PubMed] [Google Scholar]
- 32.Hino, M., Y. Nishizawa, N. Tatsumi, A. Tojo, and H. Morii. 1995. Down-modulation of c-kit mRNA and protein expression by erythroid differentiation factor/activin A. FEBS Lett. 374:69-71. [DOI] [PubMed] [Google Scholar]
- 33.Hong, L., V. Munugalavadla, and R. Kapur. 2004. c-Kit-mediated overlapping and unique functional and biochemical outcomes via diverse signaling pathways. Mol. Cell. Biol. 24:1401-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Kalfa, T. A., S. Pushkaran, J. F. Johnson, I. Wei, D. A. Williams, and Y. Zheng. 2004. Erythrocyte cytoskeletal defects induced in mice by deletion of Rac GTPase. Blood11:438a.
- 34.Kapur, R., and L. Zhang. 2001. A novel mechanism of cooperation between c-Kit and erythropoietin receptor. Stem cell factor induces the expression of Stat5 and erythropoietin receptor, resulting in efficient proliferation and survival by erythropoietin. J. Biol. Chem. 276:1099-1106. [DOI] [PubMed] [Google Scholar]
- 35.Kauffmann-Zeh, A., P. Rodriguez-Viciana, E. Ulrich, C. Gilbert, P. Coffer, J. Downward, and G. Evan. 1997. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature 385:544-548. [DOI] [PubMed] [Google Scholar]
- 36.Kent, W. J., C. W. Sugnet, T. S. Furey, K. M. Roskin, T. H. Pringle, A. M. Zahler, and D. Haussler. 2002. The human genome browser at UCSC. Genome Res. 12:996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lasota, J., J. Kopczynski, M. Majidi, M. Miettinen, and M. Sarlomo-Rikala. 2002. Apparent KIT Ser(715) deletion in GIST mRNA is not detectable in genomic DNA and represents a previously known splice variant of KIT transcript. Am. J. Pathol. 161:739-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lecuyer, E., S. Herblot, M. Saint-Denis, R. Martin, C. G. Begley, C. Porcher, S. H. Orkin, and T. Hoang. 2002. The SCL complex regulates c-kit expression in hematopoietic cells through functional interaction with Sp1. Blood 100:2430-2440. [DOI] [PubMed] [Google Scholar]
- 39.Lennartsson, J., P. Blume-Jensen, M. Hermanson, E. Ponten, M. Carlberg, and L. Ronnstrand. 1999. Phosphorylation of Shc by Src family kinases is necessary for stem cell factor receptor/c-kit mediated activation of the Ras/MAP kinase pathway and c-fos induction. Oncogene 18:5546-5553. [DOI] [PubMed] [Google Scholar]
- 40.Letting, D. L., Y. Y. Chen, C. Rakowski, S. Reedy, and G. A. Blobel. 2004. Context-dependent regulation of GATA-1 by friend of GATA-1. Proc. Natl. Acad. Sci. USA 101:476-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Letting, D. L., C. Rakowski, M. J. Weiss, and G. A. Blobel. 2003. Formation of a tissue-specific histone acetylation pattern by the hematopoietic transcription factor GATA-1. Mol. Cell. Biol. 23:1334-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li, K., C. Miller, S. Hegde, and D. Wojchowski. 2003. Roles for an Epo receptor Tyr-343 Stat5 pathway in proliferative co-signaling with kit. J. Biol. Chem. 278:40702-40709. [DOI] [PubMed] [Google Scholar]
- 43.Linnekin, D. 1999. Early signaling pathways activated by c-Kit in hematopoietic cells. Int. J. Biochem. Cell Biol. 31:1053-1074. [DOI] [PubMed] [Google Scholar]
- 44.Linnekin, D., C. S. DeBerry, and S. Mou. 1997. Lyn associates with the juxtamembrane region of c-Kit and is activated by stem cell factor in hematopoietic cell lines and normal progenitor cells. J. Biol. Chem. 272:27450-27455. [DOI] [PubMed] [Google Scholar]
- 45.Longley, B. J., L. Tyrrell, S. Z. Lu, Y. S. Ma, K. Langley, T. G. Ding, T. Duffy, P. Jacobs, L. H. Tang, and I. Modlin. 1996. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat. Genet. 12:312-314. [DOI] [PubMed] [Google Scholar]
- 46.Lux, M. L., B. P. Rubin, T. L. Biase, C. J. Chen, T. Maclure, G. Demetri, S. Xiao, S. Singer, C. D. Fletcher, and J. A. Fletcher. 2000. KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am. J. Pathol. 156:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marks, P. A., M. Sheffery, and R. A. Rifkind. 1987. Induction of transformed cells to terminal differentiation and the modulation of gene expression. Cancer Res. 47:659-666. [PubMed] [Google Scholar]
- 48.Migliaccio, A. R., R. A. Rana, M. Sanchez, R. Lorenzini, L. Centurione, L. Bianchi, A. M. Vannucchi, G. Migliaccio, and S. H. Orkin. 2003. GATA-1 as a regulator of mast cell differentiation revealed by the phenotype of the GATA-1low mouse mutant. J. Exp. Med. 197:281-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miura, O., A. D'Andrea, D. Kabat, and J. N. Ihle. 1991. Induction of tyrosine phosphorylation by the erythropoietin receptor correlates with mitogenesis. Mol. Cell. Biol. 11:4895-4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moll, J., G. Sansig, E. Fattori, and H. van der Putten. 1991. The murine rac1 gene: cDNA cloning, tissue distribution and regulated expression of rac1 mRNA by disassembly of actin microfilaments. Oncogene 6:863-866. [PubMed] [Google Scholar]
- 51.Motoyama, N., T. Kimura, T. Takahashi, T. Watanabe, and T. Nakano. 1999. bcl-x prevents apoptotic cell death of both primitive and definitive erythrocytes at the end of maturation. J. Exp. Med. 189:1691-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Motoyama, N., F. Wang, K. A. Roth, H. Sawa, K. Nakayama, I. Negishi, S. Senju, Q. Zhang, S. Fujii, et al. 1995. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267:1506-1510. [DOI] [PubMed] [Google Scholar]
- 53.Muta, K., S. B. Krantz, M. C. Bondurant, and C. H. Dai. 1995. Stem cell factor retards differentiation of normal human erythroid progenitor cells while stimulating proliferation. Blood 86:572-580. [PubMed] [Google Scholar]
- 54.Nagata, H., A. S. Worobec, C. K. Oh, B. A. Chowdhury, S. Tannenbaum, Y. Suzuki, and D. D. Metcalfe. 1995. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc. Natl. Acad. Sci. USA 92:10560-10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakata, Y., A. Kimura, O. Katoh, K. Kawaishi, H. Hyodo, K. Abe, A. Kuramoto, and Y. Satow. 1995. c-kit point mutation of extracellular domain in patients with myeloproliferative disorders. Br. J. Haematol. 91:661-663. [DOI] [PubMed] [Google Scholar]
- 56.Nichols, K. E., J. D. Crispino, M. Poncz, J. G. White, S. H. Orkin, J. M. Maris, and M. J. Weiss. 2000. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in GATA1. Nat. Genet. 24:266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nocka, K., S. Majumder, B. Chabot, P. Ray, M. Cervone, A. Bernstein, and P. Besmer. 1989. Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice—evidence for an impaired c-kit kinase in mutant mice. Genes Dev. 3:816-826. [DOI] [PubMed] [Google Scholar]
- 58.O'Hagan, R. C., M. Ohh, G. David, I. M. de Alboran, F. W. Alt, W. G. Kaelin, Jr., and R. A. DePinho. 2000. Myc-enhanced expression of Cul1 promotes ubiquitin-dependent proteolysis and cell cycle progression. Genes Dev. 14:2185-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pevny, L., C. S. Lin, V. D'Agati, M. C. Simon, S. H. Orkin, and F. Costantini. 1995. Development of hematopoietic cells lacking transcription factor GATA-1. Development 121:163-172. [DOI] [PubMed] [Google Scholar]
- 60.Pevny, L., M. C. Simon, E. Robertson, W. H. Klein, S. F. Tsai, V. D'Agati, S. H. Orkin, and F. Costantini. 1991. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature 349:257-260. [DOI] [PubMed] [Google Scholar]
- 61.Rylski, M., J. J. Welch, Y. Y. Chen, D. L. Letting, J. A. Diehl, L. A. Chodosh, G. A. Blobel, and M. J. Weiss. 2003. GATA-1-mediated proliferation arrest during erythroid maturation. Mol. Cell. Biol. 23:5031-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwartz, S., W. J. Kent, A. Smit, Z. Zhang, R. Baertsch, R. C. Hardison, D. Haussler, and W. Miller. 2003. Human-mouse alignments with BLASTZ. Genome Res. 13:103-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sears, R., F. Nuckolls, E. Haura, Y. Taya, K. Tamai, and J. R. Nevins. 2000. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 14:2501-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serve, H., N. S. Yee, G. Stella, L. Sepp-Lorenzino, J. C. Tan, and P. Besmer. 1995. Differential roles of PI3-kinase and Kit tyrosine 821 in Kit receptor-mediated proliferation, survival and cell adhesion in mast cells. EMBO J. 14:473-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimizu, R., T. Kuroha, O. Ohneda, X. Pan, K. Ohneda, S. Takahashi, S. Philipsen, and M. Yamamoto. 2004. Leukemogenesis caused by incapacitated GATA-1 function. Mol. Cell. Biol. 24:10814-10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shirsat, N. V., R. J. Pignolo, B. L. Kreider, and G. Rovera. 1990. A member of the ras gene superfamily is expressed specifically in T, B and myeloid hemopoietic cells. Oncogene 5:769-772. [PubMed] [Google Scholar]
- 67.Shivdasani, R. A., Y. Fujiwara, M. A. McDevitt, and S. H. Orkin. 1997. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 16:3965-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Socolovsky, M., A. E. Fallon, S. Wang, C. Brugnara, and H. F. Lodish. 1999. Fetal anemia and apoptosis of red cell progenitors in Stat5a−/−5b−/− mice: a direct role for Stat5 in Bcl-X(L) induction. Cell 98:181-191. [DOI] [PubMed] [Google Scholar]
- 69.Takahashi, S., T. Komeno, N. Suwabe, K. Yoh, O. Nakajima, S. Nishimura, T. Kuroha, T. Nagasawa, and M. Yamamoto. 1998. Role of GATA-1 in proliferation and differentiation of definitive erythroid and megakaryocytic cells in vivo. Blood 92:434-442. [PubMed] [Google Scholar]
- 70.Tan, B. L., L. Hong, V. Munugalavadla, and R. Kapur. 2003. Functional and biochemical consequences of abrogating the activation of multiple diverse early signaling pathways in Kit. Role for Src kinase pathway in Kit-induced cooperation with erythropoietin receptor. J. Biol. Chem. 278:11686-11695. [DOI] [PubMed] [Google Scholar]
- 71.Tan, B. L., M. N. Yazicioglu, D. Ingram, J. McCarthy, J. Borneo, D. A. Williams, and R. Kapur. 2003. Genetic evidence for convergence of c-Kit- and alpha4 integrin-mediated signals on class IA PI-3kinase and the Rac pathway in regulating integrin-directed migration in mast cells. Blood 101:4725-4732. [DOI] [PubMed] [Google Scholar]
- 72.Timokhina, I., H. Kissel, G. Stella, and P. Besmer. 1998. Kit signaling through PI 3-kinase and Src kinase pathways: an essential role for Rac1 and JNK activation in mast cell proliferation. EMBO J. 17:6250-6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Twamley-Stein, G. M., R. Pepperkok, W. Ansorge, and S. A. Courtneidge. 1993. The Src family tyrosine kinases are required for platelet-derived growth factor-mediated signal transduction in NIH 3T3 cells. Proc. Natl. Acad. Sci. USA 90:7696-7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uddin, S., S. Kottegoda, D. Stigger, L. C. Platanias, and A. Wickrema. 2000. Activation of the Akt/FKHRL1 pathway mediates the antiapoptotic effects of erythropoietin in primary human erythroid progenitors. Biochem. Biophys. Res. Commun. 275:16-19. [DOI] [PubMed] [Google Scholar]
- 75.Ueda, S., M. Mizuki, H. Ikeda, T. Tsujimura, I. Matsumura, K. Nakano, H. Daino, Z.-I. Honda, J. Sonoyama, H. Shibayama, H. Sugahara, T. Machii, and Y. Kanakura. 2002. Critical roles of c-Kit tyrosine residues 567 and 719 in stem cell factor-induced chemotaxis: contribution of src family kinase and PI3-kinase on calcium mobilization and cell migration. Blood 99:3342-3349. [DOI] [PubMed] [Google Scholar]
- 76.van der Geer, P., T. Hunter, and R. A. Lindberg. 1994. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu. Rev. Cell Biol. 10:251-337. [DOI] [PubMed] [Google Scholar]
- 77.Vannucchi, A. M., L. Bianchi, C. Cellai, F. Paoletti, V. Carrai, A. Calzolari, L. Centurione, R. Lorenzini, C. Carta, E. Alfani, M. Sanchez, G. Migliaccio, and A. R. Migliaccio. 2001. Accentuated response to phenylhydrazine and erythropoietin in mice genetically impaired for their GATA-1 expression (GATA-1(low) mice). Blood 97:3040-3050. [DOI] [PubMed] [Google Scholar]
- 78.Vannucchi, A. M., L. Bianchi, C. Cellai, F. Paoletti, R. A. Rana, R. Lorenzini, G. Migliaccio, and A. R. Migliaccio. 2002. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1(low) mice). Blood 100:1123-1132. [DOI] [PubMed] [Google Scholar]
- 79.Weiss, M. J., G. Keller, and S. H. Orkin. 1994. Novel insights into erythroid development revealed through in vitro differentiation of GATA-1 embryonic stem cells. Genes Dev. 8:1184-1197. [DOI] [PubMed] [Google Scholar]
- 80.Weiss, M. J., and S. H. Orkin. 1995. Transcription factor GATA-1 permits survival and maturation of erythroid precursors by preventing apoptosis. Proc. Natl. Acad. Sci. USA 92:9623-9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weiss, M. J., C. Yu, and S. H. Orkin. 1997. Erythroid-cell-specific properties of transcription factor GATA-1 revealed by phenotypic rescue of a gene-targeted cell line. Mol. Cell. Biol. 17:1642-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Welch, J. J., J. A. Watts, C. R. Vakoc, Y. Yao, H. Wang, R. C. Hardison, G. A. Blobel, L. A. Chodosh, and M. J. Weiss. 2004. Global regulation of erythroid gene expression by transcription factor GATA-1. Blood 104:3136-3147. [DOI] [PubMed] [Google Scholar]
- 83.Wessely, O., G. Mellitzer, M. von Lindern, A. Levitzki, A. Gazit, I. Ischenko, M. J. Hayman, and H. Beug. 1997. Distinct roles of the receptor tyrosine kinases c-ErbB and c-Kit in regulating the balance between erythroid cell proliferation and differentiation. Cell Growth Differ. 8:481-493. [PubMed] [Google Scholar]
- 84.Wingender, E., X. Chen, E. Fricke, R. Geffers, R. Hehl, I. Liebich, M. Krull, V. Matys, H. Michael, R. Ohnhauser, M. Pruss, F. Schacherer, S. Thiele, and S. Urbach. 2001. The TRANSFAC system on gene expression regulation. Nucleic Acids Res. 29:281-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu, G., M. Nagano, R. Kanezaki, T. Toki, Y. Hayashi, T. Taketani, T. Taki, T. Mitui, K. Koike, K. Kato, M. Imaizumi, I. Sekine, Y. Ikeda, R. Hanada, M. Sako, K. Kudo, S. Kojima, O. Ohneda, M. Yamamoto, and E. Ito. 2003. Frequent mutations in the GATA-1 gene in the transient myeloproliferative disorder of Down syndrome. Blood 102:2960-2968. [DOI] [PubMed] [Google Scholar]
- 86.Yang, F. C., R. Kapur, A. J. King, W. Tao, C. Kim, J. Borneo, R. Breese, M. Marshall, M. C. Dinauer, and D. A. Williams. 2000. Rac2 stimulates Akt activation affecting BAD/Bcl-XL expression while mediating survival and actin function in primary mast cells. Immunity 12:557-568. [DOI] [PubMed] [Google Scholar]
- 87.Yu, C., A. B. Cantor, H. Yang, C. Browne, R. A. Wells, Y. Fujiwara, and S. H. Orkin. 2002. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med. 195:1387-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu, C., K. K. Niakan, M. Matsushita, G. Stamatoyannopoulos, S. H. Orkin, and W. H. Raskind. 2002. X-linked thrombocytopenia with thalassemia from a mutation in the amino finger of GATA-1 affecting DNA binding rather than FOG-1 interaction. Blood 100:2040-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]