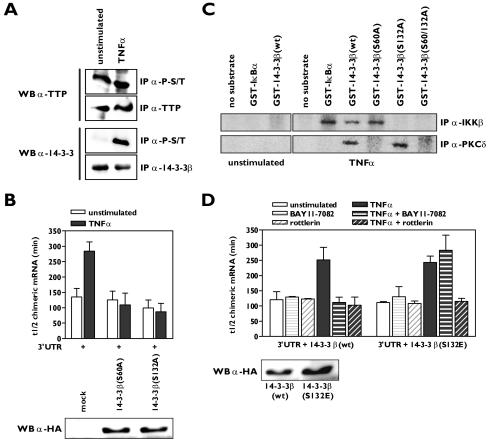
FIG. 5.
Phosphorylation of 14-3-3β by IKKβ and PKCδ promotes β4GalT1 mRNA stabilization. (A) TNF-α stimulation induces serine/threonine phosphorylation of 14-3-3β, while TTP is already heavily phosphorylated on serine/threonine residues in resting HUVECs. Phospho-serine/threonine (P-S/T)-containing proteins were immunoprecipitated from lysates of unstimulated HUVECs or HUVECs stimulated for 15 min with TNF-α. Western blotting was performed using anti-TTP and anti-14-3-3β antibodies. Total levels of TTP and 14-3-3β were also checked by control immunoprecipitations. (B) 14-3-3β mutants carrying S60A or S132A substitutions interfere with TNF-α-induced β4GalT1 mRNA stabilization. 14-3-3β(S60A) or 14-3-3β(S132A) was overexpressed in HeLa TO cells expressing chimeric mRNA containing the wt β4GalT1 3′UTR. Its half-life was determined as described in the legend to Fig. 1C. The graphs (relative chimeric mRNA expression versus time after the addition of DOX) for the determination of the half-lives are available in Fig. S5A in the supplemental material. The overexpression of HA-tagged 14-3-3β(S60A) and 14-3-3β(S132A) was checked by Western blotting with an anti-HA antibody as described in Materials and Methods. (C) Ser60 and Ser132 of 14-3-3β are phosphorylated by PKCδ and IKKβ in vitro. PKCδ and IKKβ were immunoprecipitated from lysates of u nstimulated HUVECs or HUVECs stimulated for 15 min with TNF-α. Bacterially expressed GST-14-3-3β fusion proteins were used as substrates in kinase assays. (D) Acquired resistance to rottlerin in 14-3-3β(S132E)-expressing cells with regard to TNF-α-induced β4GalT1 mRNA stabilization. 14-3-3β(S132E) was overexpressed in HeLa TO cells expressing chimeric mRNA containing the wt β4GalT1 3′UTR. The cells were treated with BAY11-7082 or rottlerin as indicated, before the half-life of the chimeric mRNA was determined as described in the legend to Fig. 1C. The graphs (relative chimeric mRNA expression versus time after the addition of DOX) for the determination of the half-lives are available in Fig. S5B in the supplemental material. The overexpression of HA-tagged 14-3-3β(wt) and 14-3-3β(S132E) was checked by Western blotting with an anti-HA antibody as described in Materials and Methods.