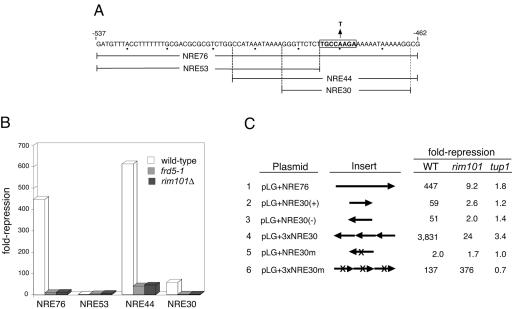
FIG. 1.
A RIM101-dependent operator element within NREDIT. (A) The sequence of the 76-bp NREDIT, from nt −537 to −462 upstream of the DIT1 gene, is given in the top line and denoted as NRE76. The PacCDIT sequence is boxed and annotated to show the A-to-T mutation at nt −480. The sequences spanned by NRE53, NRE44, and NRE30 are also shown. (B) Strains W303-1A (wild type, open bars), Y108 (frd5-1, gray bars), and Y104 (rim101Δ, black bars) were separately transformed with pLG312, pLG+NRE76, pLG+NRE53, and pLG+NRE30(+). Repression (n-fold) of gene expression is given as the ratio of β-galactosidase activity measured in crude extracts of cells in which the plasmid-borne CYC1-lacZ reporter gene had no insert to the activity measured in cells from the same strain in which the plasmid-borne CYC1-lacZ reporter gene contained the indicated NRE site inserted between the UASCYC1 and the TATA box (see Materials and Methods). (C) Strains W303-1A (wild type [WT]), Y104 (rim101Δ), and Y170 (tup1Δ) were separately transformed with each of the plasmids indicated in the first column and pLG312. The arrowheads in the schematic of the inserts given in the second column denote the orientation of the inserts in the plasmid-borne CYC1-lacZ reporter genes relative to the DIT1 gene. NRE30m and 3×NRE30m are identical to NRE30(−) and 3×NRE30, respectively, with the exception that the A residue at nt −480 has been mutated to a T residue and the orientation of the inserts is reversed in 3×NRE30m relative to 3×NRE30. Repression (n-fold) given in the last three columns refers to the ratio of β-galactosidase activity measured in cells containing pLG312 to the activity measured in cells of the same strain containing the plasmid-borne CYC1-lacZ reporter with the indicated insert. The activities reported in this figure and in Fig. 2 are the average activities obtained from three or more independent cultures analyzed at the same time. Each experiment was repeated from one to five times; although absolute values varied between experiments, the relative levels of β-galactosidase activities were similar.