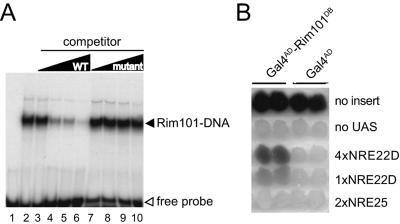
FIG. 3.
Rim101 binds to the PacCDIT site in vitro and in vivo. (A) A truncated version of Rim101 extending from residue 1 to residue 289 was synthesized in vitro and tested for its ability to bind to a radiolabeled NRE22D-containing oligonucleotide by EMSA as described in Materials and Methods. An equivalent amount of Rim101(1-289) protein was incubated with a radiolabeled NRE22D-containing double-stranded oligonucleotide (5′-GATCCTTGCCAAGAAAAAATAAAAAGGATC-3′ [the PacCDIT site is shown in boldface type]) in the absence of competitor DNA (lane 2) and in the presence of a 10-, 50-, 100-, or 250-fold excess of nonlabeled NRE22D-containing double-stranded oligonucleotide (lanes 3 to 6) or a nonlabeled mutant version of this double-stranded oligonucleotide (lanes 7 to 10) as specific and nonspecific competitors, respectively. The mutant oligonucleotide had a 1-bp change within the PacCDIT site, changing the sequence from 5′-TGCCAAGA-3′ to 5′-TGCCTAGA-3′. The reaction of lane 1 contained only the probe DNA. WT, wild type. (B) A Gal4AD-Rim101(1-289) fusion protein expressed in vivo activates an NRE22D-containing reporter gene. W303-1B was transformed with pACTII-Rim101(1-289) (Gal4AD-Rim101DB) or pACTII (Gal4AD), as indicated at the top of the panel. As indicated on the right of the panel, these transformants were then transformed with pLG312 (no insert); pLGΔSS (no UAS); pLGΔSS+4×NRE22D, which contains a 4×NRE22D-lacZ gene reporter gene; pLGΔSS+1×NRE22D, which contains a 1×NRE22D-lacZ reporter gene; or pLGΔSS+2×NRE25, which contains a 2×NRE25-lacZ reporter gene. Colonies representing two distinct transformants of each strain were grown up and overlaid with X-Gal-containing agar to monitor β-galactosidase expression.