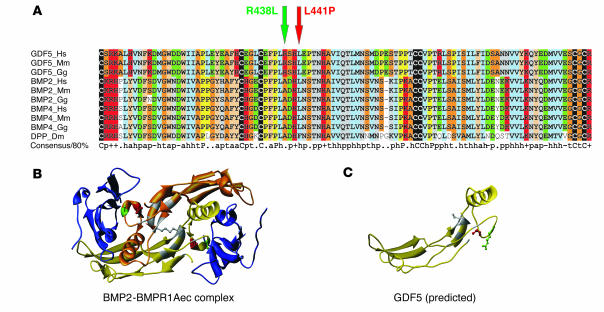
Figure 2.
Protein sequence alignment of GDF5 homologs and 3D models for GDF5 receptor binding. (A) Primary sequence alignment of GDF5, BMP2, and BMP4 from different species, including drosophila DPP. The positions of R438L and L441P mutations in GDF5 are indicated by arrows. Note the widespread conservation of residue L441 in BMPs and the specificity of R438 conservation for the GDF5 subfamily (replaced by A in the BMPs). (B) 3D model of a BMP2 dimer (yellow and gold) linked via a disulfide bridge and bound to the ectodomain of BMPR1A (BMPR1Aec). The amino acids mutated in GDF5 are indicated in red (L441) and green (R438). Note their position within the receptor interaction site. (C) Predicted structure of a GDF5 monomer. The positions of the mutated amino acids are indicated in red (L441) and green (R438).