Abstract
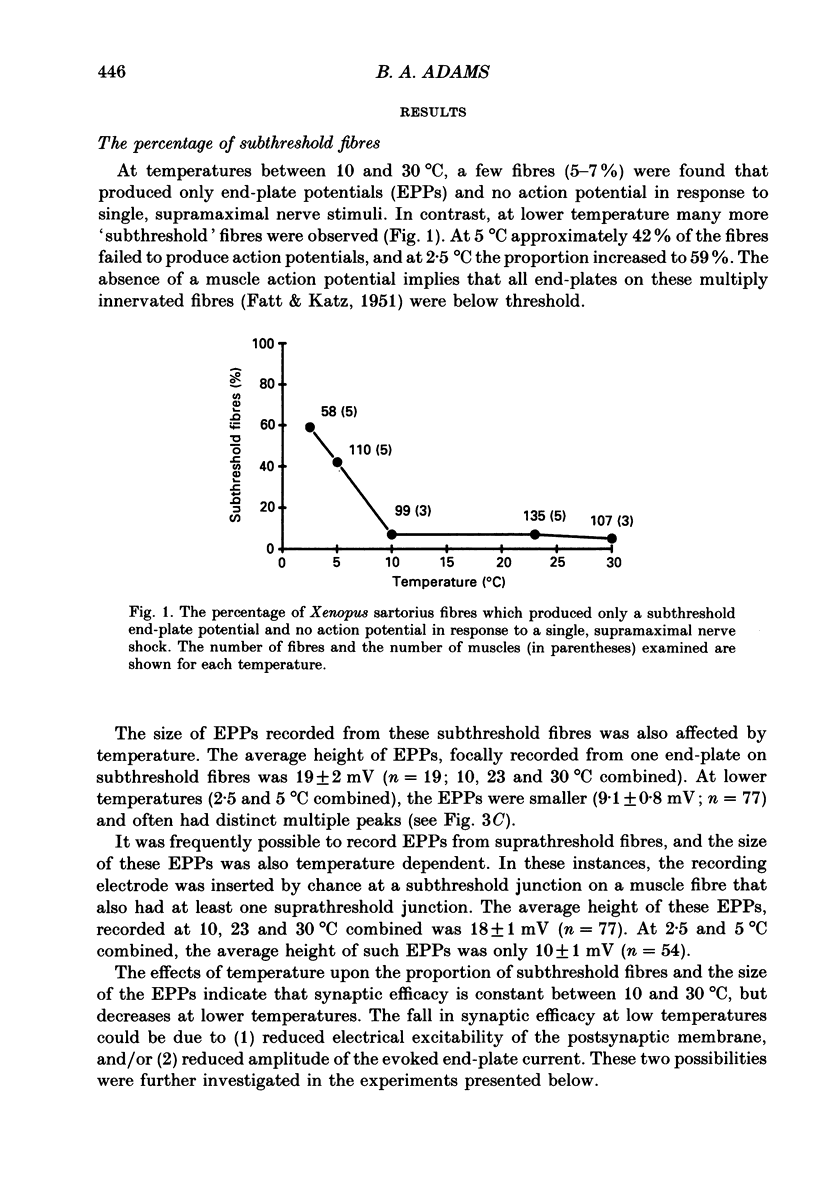
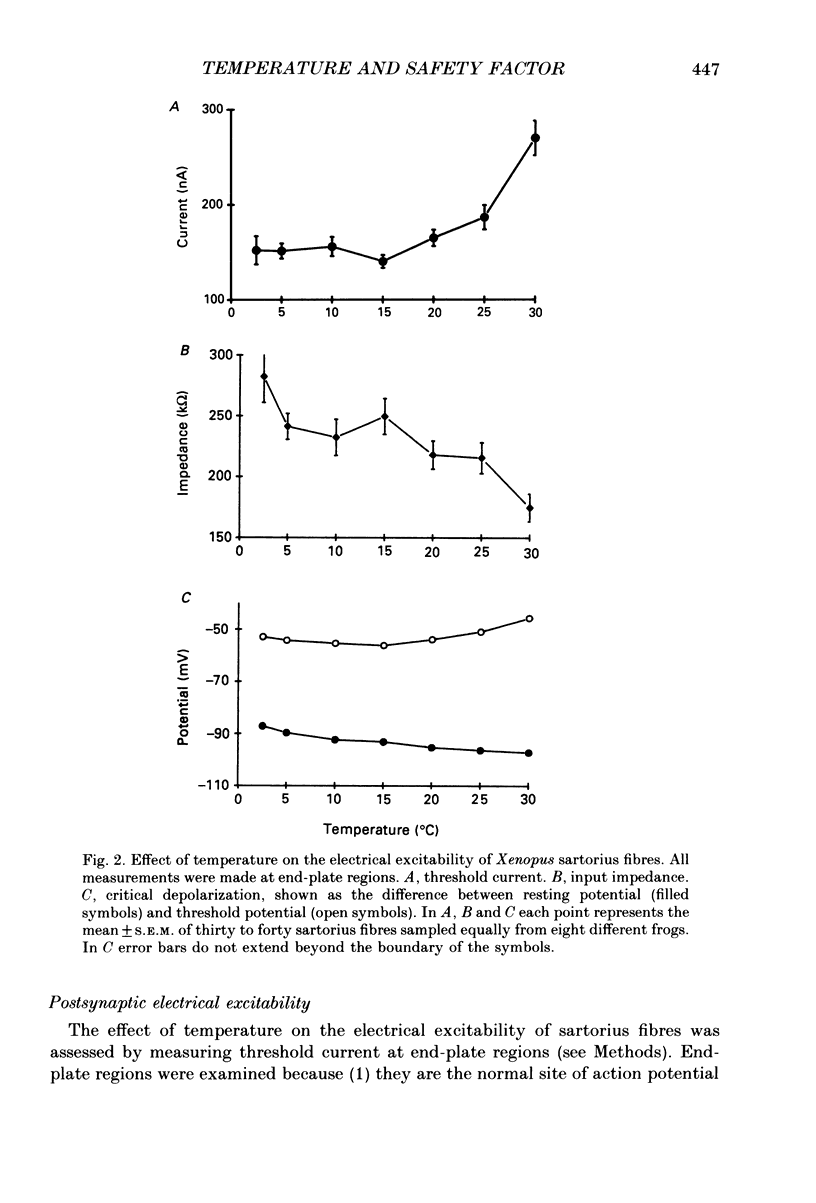
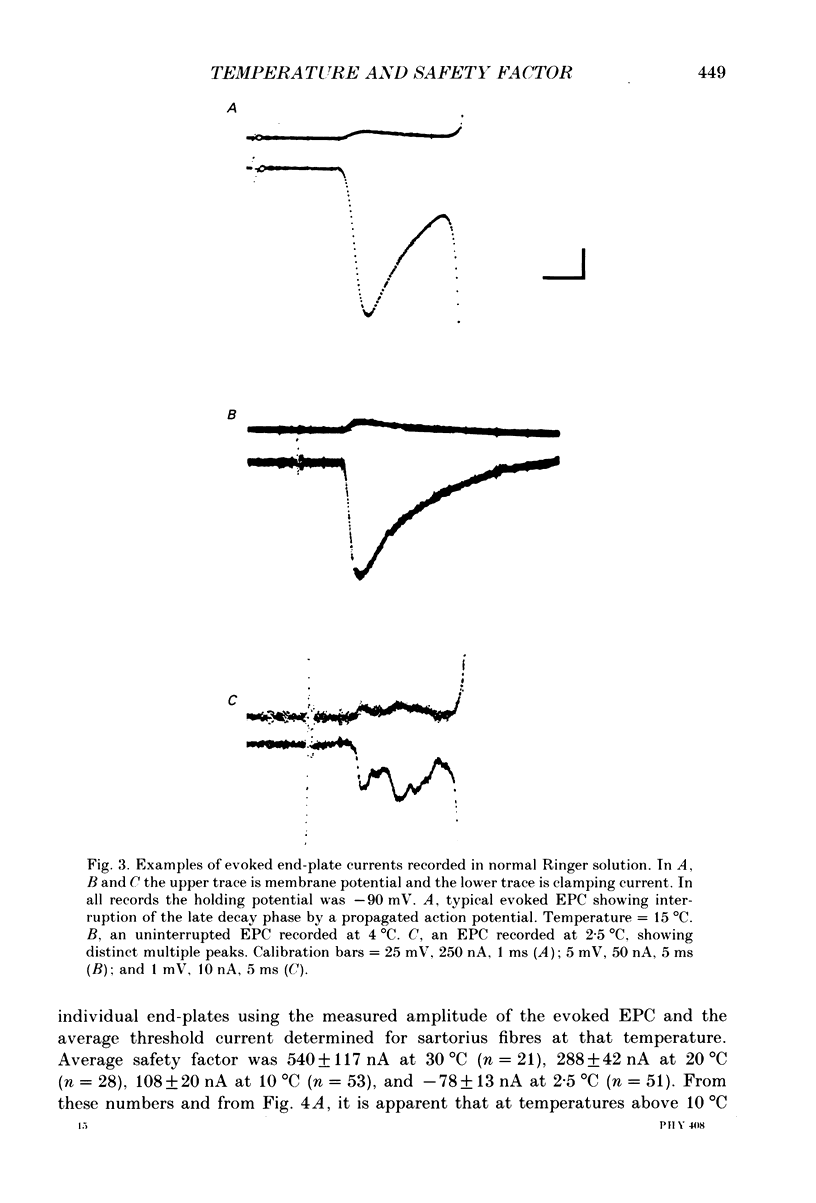
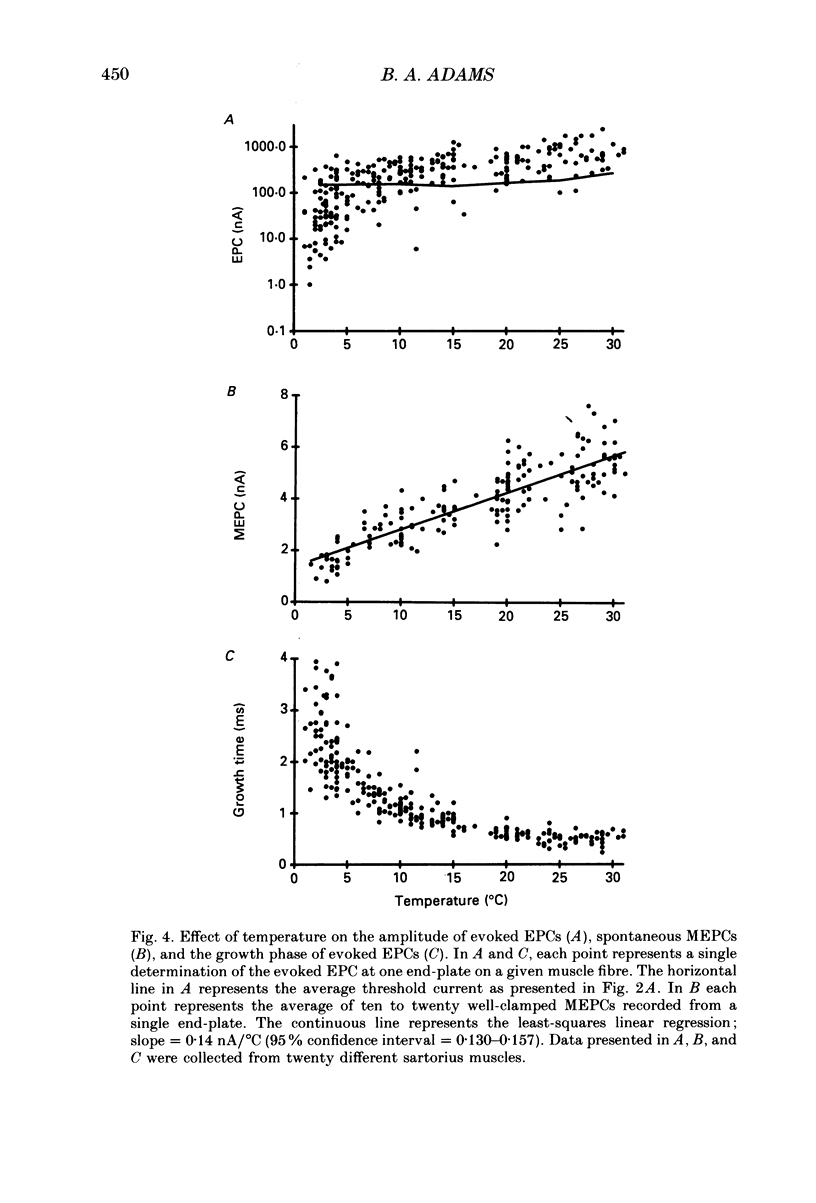
1. Intracellular recording and voltage-clamp techniques were used to measure synaptic efficacy and the safety factor for neuromuscular transmission in frog skeletal muscle. All measurements were made in normal Ringer solution, in the absence of presynaptic or postsynaptic blocking agents. 2. Over a broad temperature range (10-30 degrees C), a small percentage of sartorius fibres (about 6%) could be found which produced only subthreshold end-plate potentials and no action potential in response to single, supramaximal nerve shock. At lower temperatures the proportion of such fibres increased; 42% of the fibres had subthreshold transmission at 5 degrees C, and 59% were subthreshold at 2.5 degrees C. 3. Threshold current, measured by intracellularly injecting short pulses of depolarizing current at end-plate regions, was independent of temperature between 2.5 and 20 degrees C. Thus, the reduced synaptic efficacy observed at low temperatures was not due to decreased electrical excitability of the postsynaptic membrane. 4. The amplitude of evoked end-plate currents (EPCs) decreased with cooling. At temperatures below 10 degrees C, the evoked EPCs at many end-plates were too small to initiate action potentials. The decline in EPC amplitude was due to three factors: a decrease in the amplitude of single quantum currents (MEPCs), an increase in the temporal dispersion of transmitter release, and (below 5 degrees C) a decrease in quantal content. 5. The safety factor for neuromuscular transmission decreased dramatically as temperature was lowered. At 30 degrees C average safety factor was large and positive (540 nA), but at 2.5 degrees C it was negative (-78 nA). 6. The quantal content of evoked transmitter release was independent of temperature change between 5 and 30 degrees C, the average value over this range being 180. However, at temperatures below 5 degrees C, quantal content fell off sharply (average value = 37). 7. The thermal independence of transmitter release may be an important mechanism in allowing poikilothermic animals to maintain physiological function over a wide range of body temperatures.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beam K. G. A voltage-clamp study of the effect of two lidocaine derivatives on the time course of end-plate currents. J Physiol. 1976 Jun;258(2):279–300. doi: 10.1113/jphysiol.1976.sp011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G., Caldwell J. H., Campbell D. T. Na channels in skeletal muscle concentrated near the neuromuscular junction. Nature. 1985 Feb 14;313(6003):588–590. doi: 10.1038/313588a0. [DOI] [PubMed] [Google Scholar]
- Caldwell J. H., Campbell D. T., Beam K. G. Na channel distribution in vertebrate skeletal muscle. J Gen Physiol. 1986 Jun;87(6):907–932. doi: 10.1085/jgp.87.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Atwood H. L. Synaptic transmission: temperature-sensitivity of calcium entry in presynaptic terminals. Brain Res. 1979 Jul 20;170(3):543–546. doi: 10.1016/0006-8993(79)90972-7. [DOI] [PubMed] [Google Scholar]
- Eusebi F., Miledi R. Divalent cations and temperature dependent block of impulse propagation at the frog neuromuscular junction. Muscle Nerve. 1983 Oct;6(8):602–605. doi: 10.1002/mus.880060811. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Miniature end-plate currents and potentials generated by quanta of acetylcholine in glycerol-treated toad sartorius fibres. J Physiol. 1972 Oct;226(1):79–94. doi: 10.1113/jphysiol.1972.sp009974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell A. D., Herrera A. A. Physiological regulation of synaptic effectiveness at frog neuromuscular junctions. J Physiol. 1980 Oct;307:301–317. doi: 10.1113/jphysiol.1980.sp013436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner R. W. Temperature effects on neuronal elements. Fed Proc. 1981 Dec;40(14):2814–2818. [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965 Dec;181(3):656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C. L., GOURAS P. Effect of cooling on neuromuscular transmission in the frog. Am J Physiol. 1958 Mar;192(3):464–470. doi: 10.1152/ajplegacy.1958.192.3.464. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. Spontaneous release of transmitter substance in multiquantal units. J Physiol. 1957 May 23;136(3):595–605. doi: 10.1113/jphysiol.1957.sp005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Nikolsky E. E., Giniatullin R. A. End-plate currents evoked by paired stimuli in frog muscle fibres. Pflugers Arch. 1984 Jun;401(2):185–192. doi: 10.1007/BF00583880. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Dreyer F., Peper K. Current-voltage relation and reversal potential at junctional and extrajunctional ACh-receptors of the frog neuromuscular junction. Pflugers Arch. 1976 Mar 11;362(1):43–47. doi: 10.1007/BF00588679. [DOI] [PubMed] [Google Scholar]
- Marsh R. L., Bennett A. F. Thermal dependence of isotonic contractile properties of skeletal muscle and sprint performance of the lizard Dipsosaurus dorsalis. J Comp Physiol B. 1985;155(5):541–551. doi: 10.1007/BF00694443. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Effects of strontium ions on end-plate channel properties. J Physiol. 1980 Sep;306:567–577. doi: 10.1113/jphysiol.1980.sp013415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge R. M., Thomson A. M. Electrical responses of muscle fibres in a small foot muscle of Xenopus laevis. J Physiol. 1980 Sep;306:41–49. doi: 10.1113/jphysiol.1980.sp013384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M. Sodium channels near end-plates and nuclei of snake skeletal muscle. J Physiol. 1987 Jul;388:213–232. doi: 10.1113/jphysiol.1987.sp016611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- Thesleff S., Vyskocil F., Ward M. R. The action potential in end-plate and extrajunctional regions of rat skeletal muscle. Acta Physiol Scand. 1974 Jun;91(2):196–202. doi: 10.1111/j.1748-1716.1974.tb05676.x. [DOI] [PubMed] [Google Scholar]


