Abstract
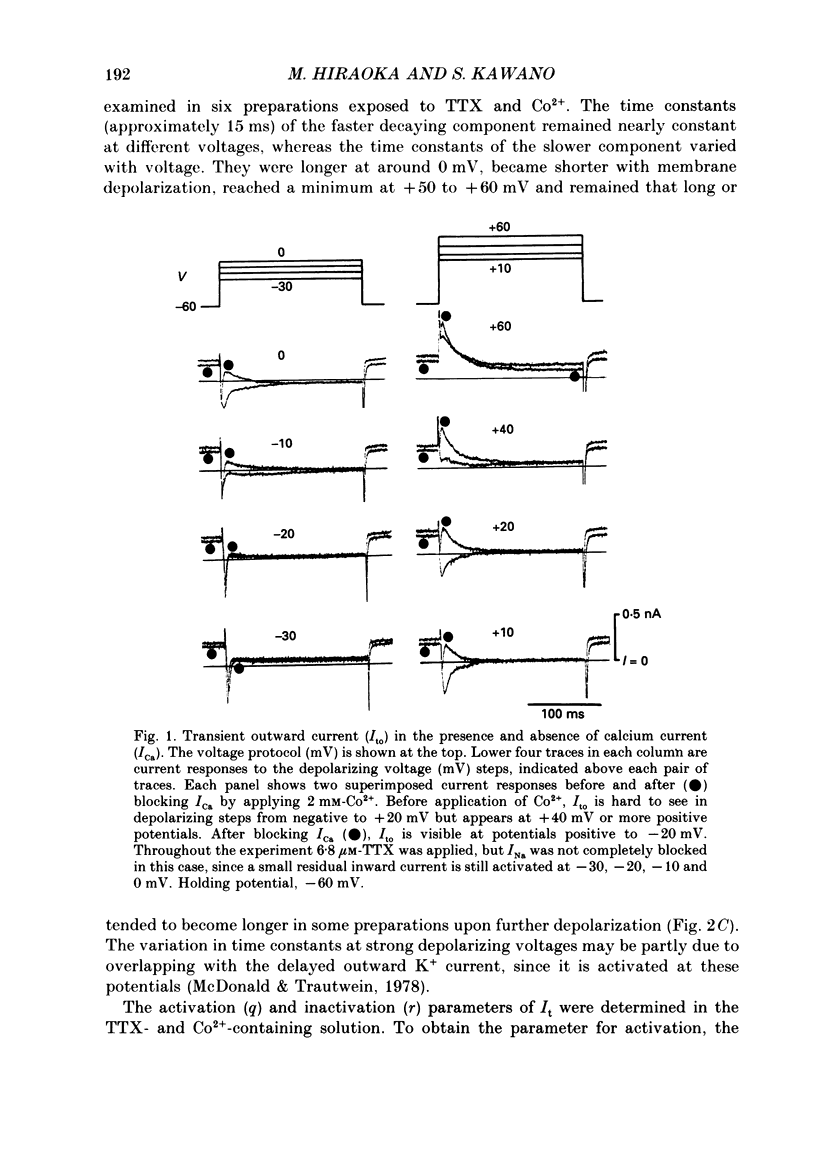
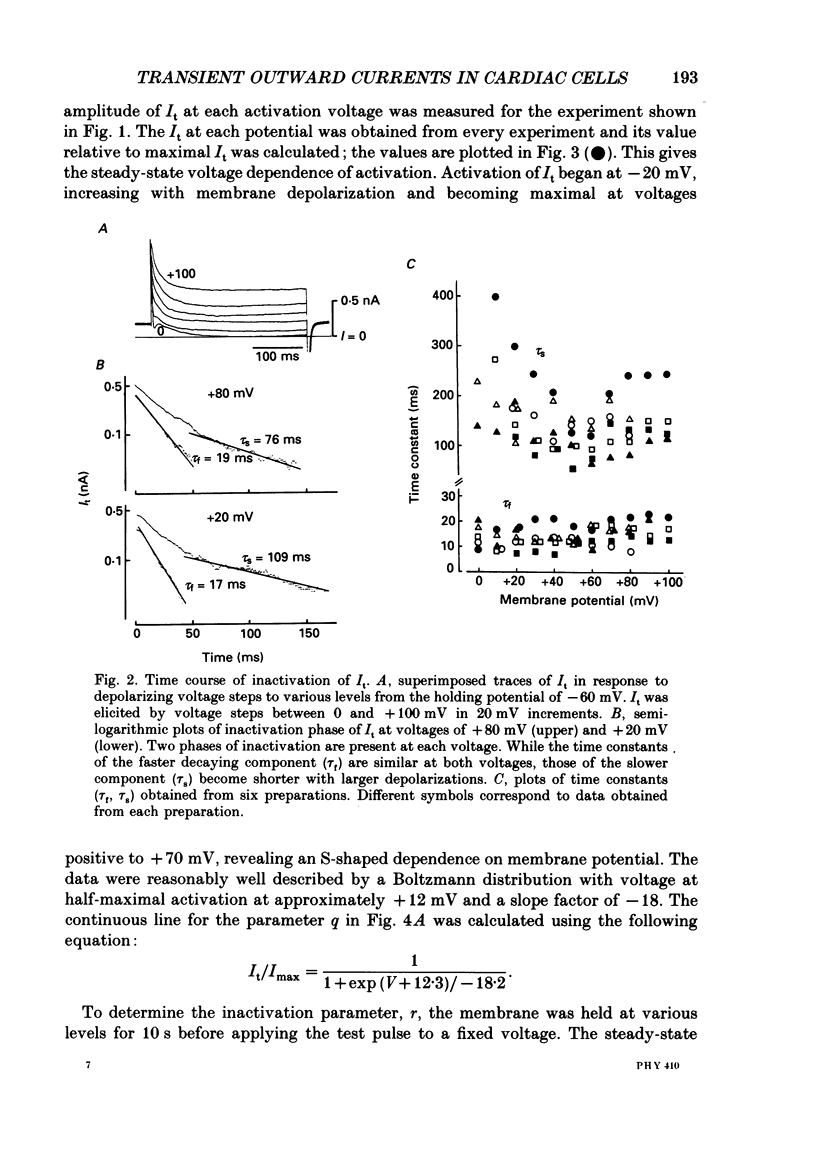
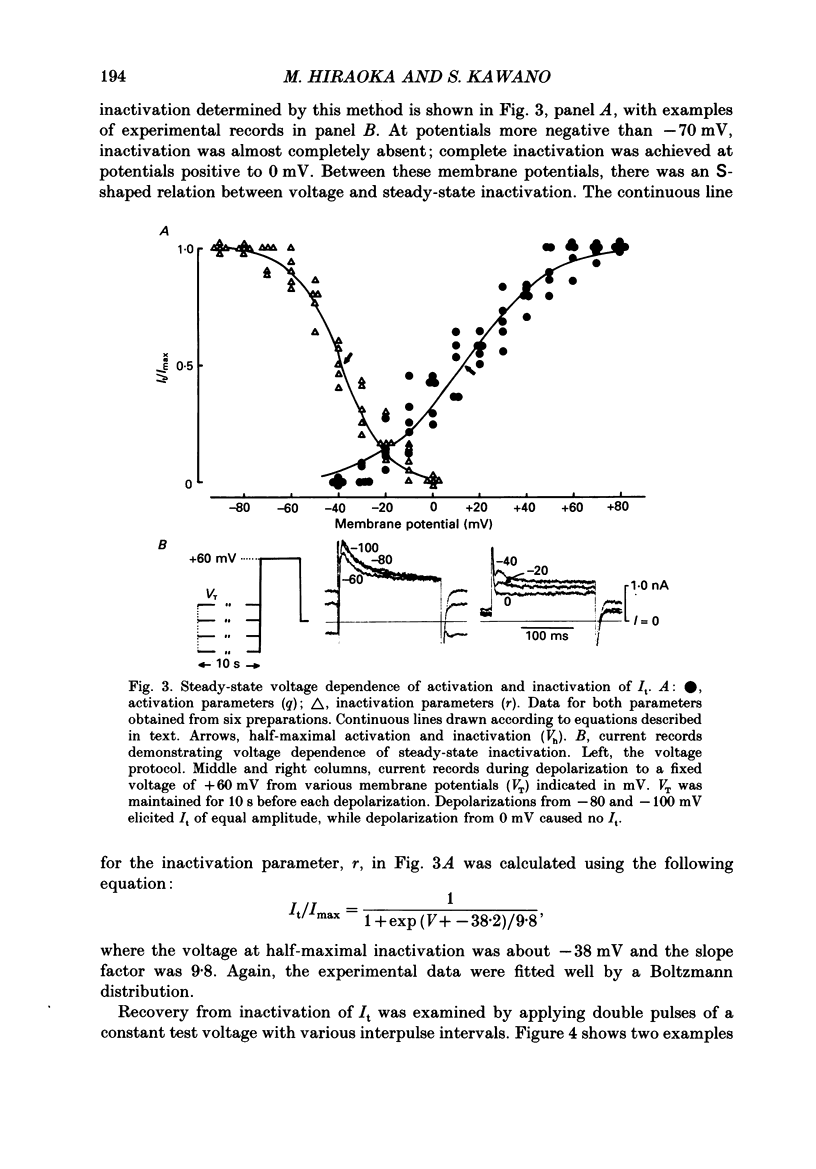
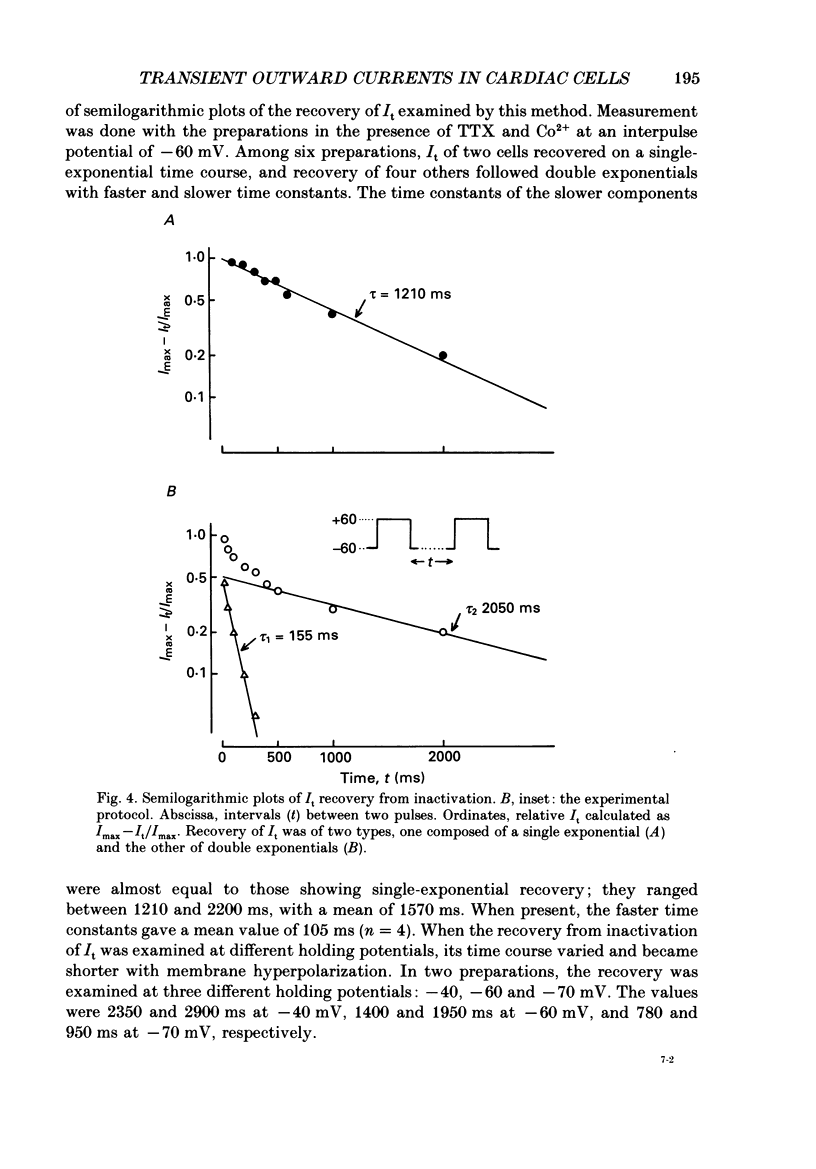
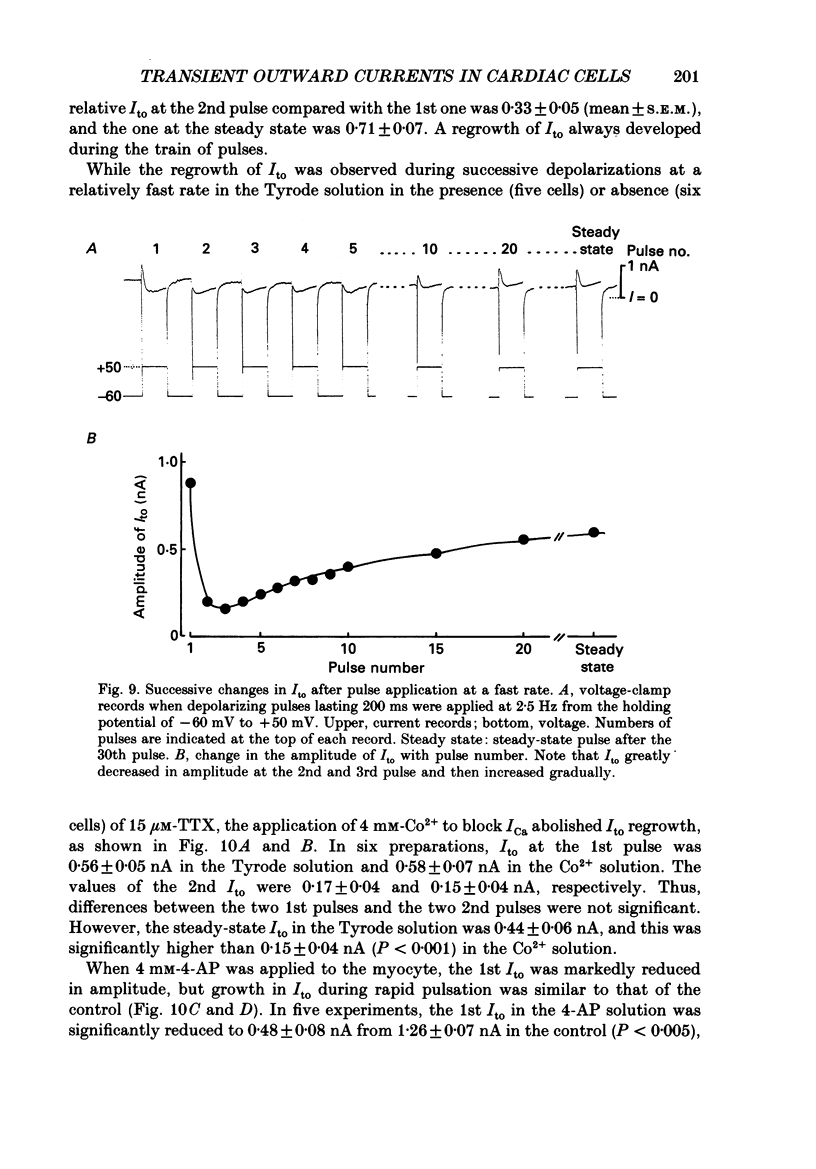
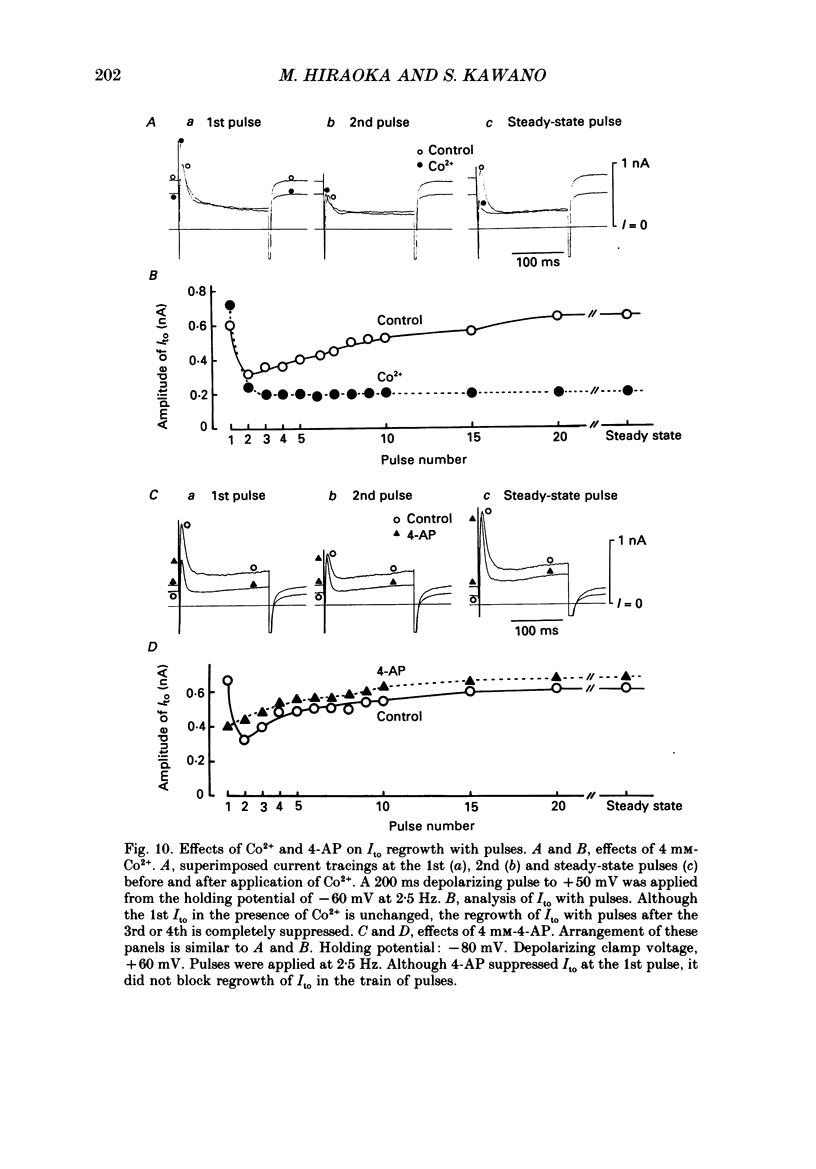
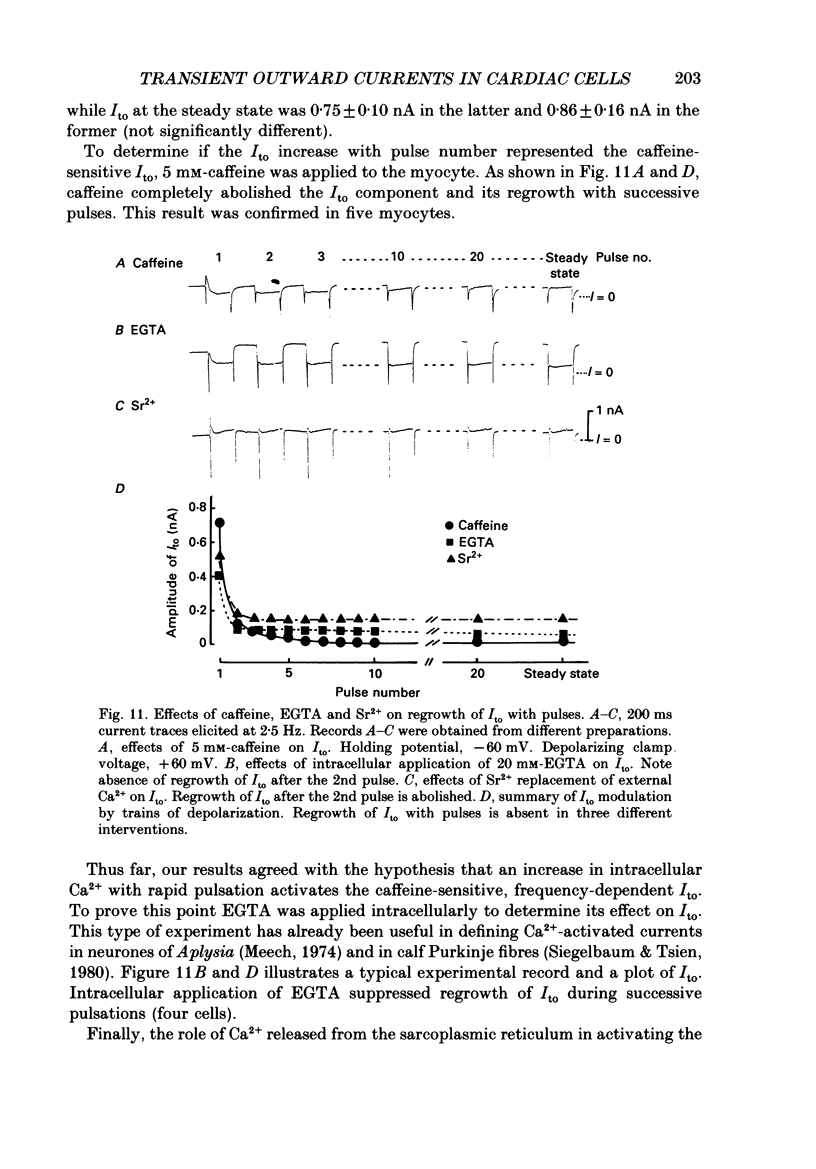
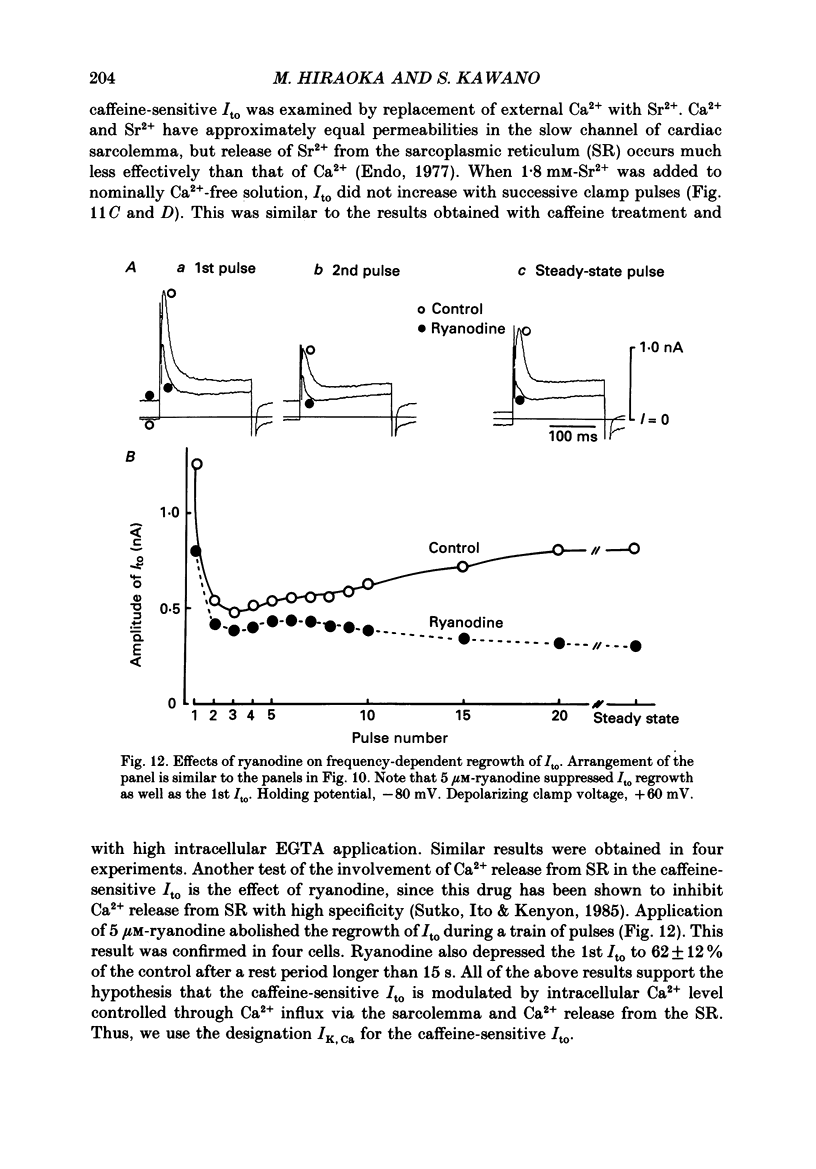
1. A suction pipette whole-cell voltage-clamp technique was used to record membrane currents and potentials of isolated ventricular myocytes from rabbit hearts. 2. Transient outward current (Ito) was activated by voltage steps positive to -20 mV, increasing in amplitude with further depolarization to reach a maximum around +70 mV. The current attained its peak within 10 ms and then it inactivated for 100-200 ms. 3. A large portion of Ito still remained after the calcium current (ICa) was blocked when depolarizing pulses were applied at a frequency of 0.1 Hz or less. Therefore, this current component is referred to as calcium-insensitive Ito or It. 4. It showed voltage- and time-dependent inactivation similar to that observed in Purkinje fibres and other cardiac preparations. 5. The reversal potential of It depended on external K+ concentration, [K+]o, with a slope of 32 mV per 10-fold change in the presence of a normal [Na+]o (143 mM), while the slope was 48 mV per 10-fold change in low [Na+]o (1.0 mM). 6. It was completely inhibited by 2-4 mM-4-aminopyridine. Ito in the presence of ICa was also partially blocked by 4-aminopyridine and the remainder was abolished by 5 mM-caffeine. 7. The calcium-insensitive and caffeine-sensitive Ito differed in their decay rates as well as in their recovery time courses. The former was predominantly available at a slow pulsing rate, while the latter increased its amplitude with high-frequency depolarization. 8. The caffeine-sensitive Ito was inhibited by a blockade of ICa, by replacing Ca2+ with Sr2+, by external application of ryanodine and by internal application of EGTA. This indicates that the current is calcium-sensitive and is dependent on increased myoplasmic Ca2+ through Ca2+ influx via the sarcolemma and Ca2+ release from the sarcoplasmic reticulum. The current is therefore designated as IK, Ca. 9. The physiological functions of IK, Ca and It are indicated by their contribution to ventricular repolarization at fast and slow heart rates, respectively.
Full text
PDF












Selected References
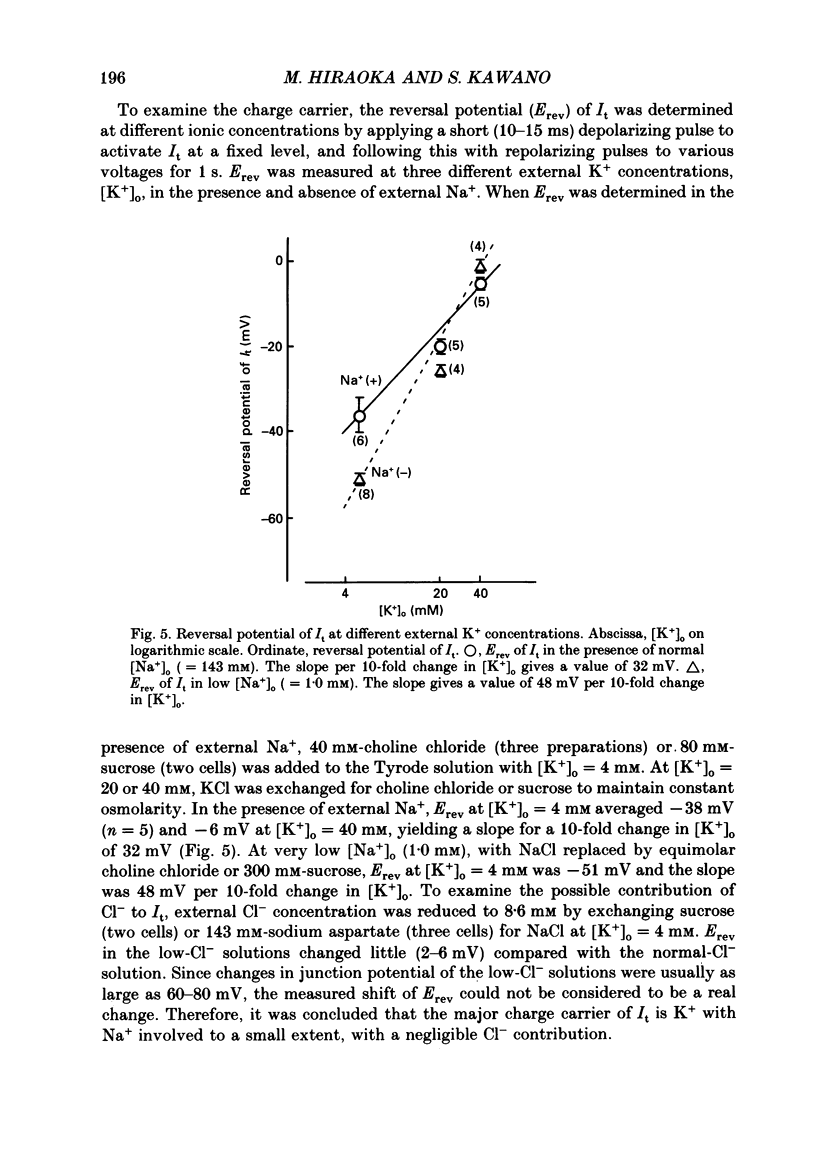
These references are in PubMed. This may not be the complete list of references from this article.
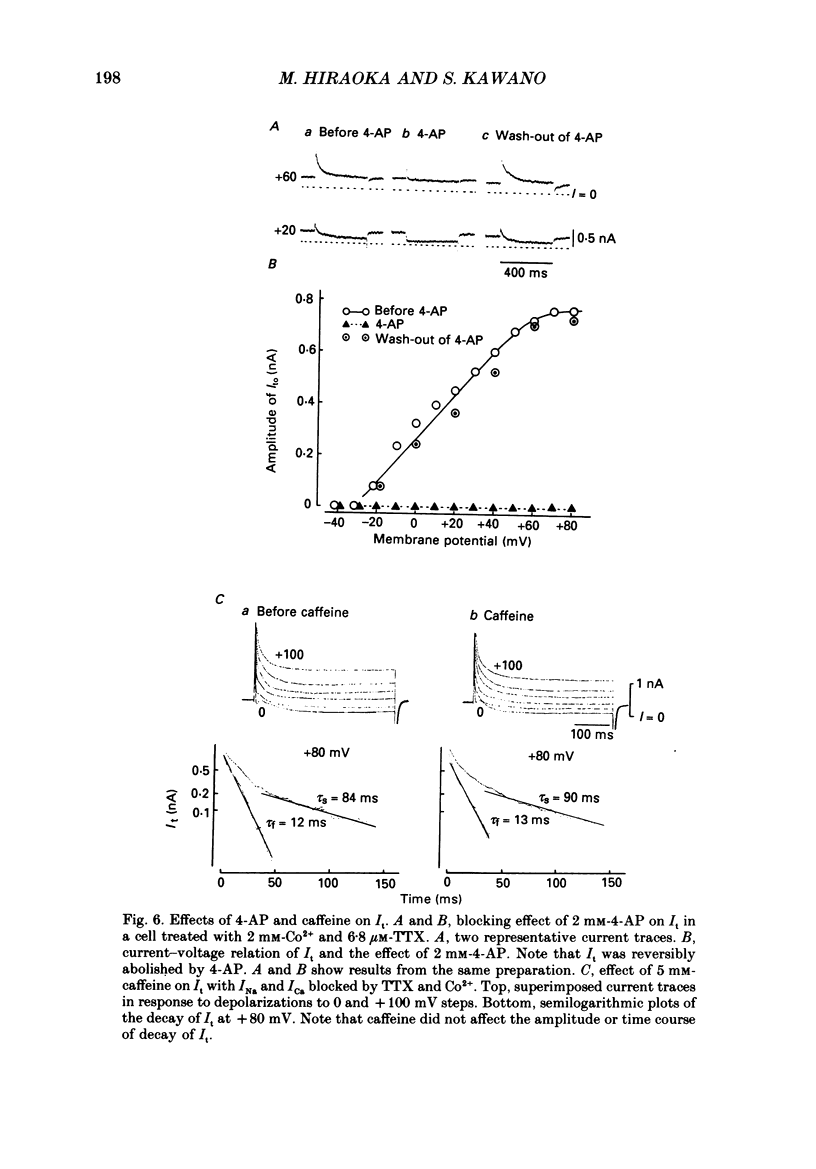
- Adams D. J., Smith S. J., Thompson S. H. Ionic currents in molluscan soma. Annu Rev Neurosci. 1980;3:141–167. doi: 10.1146/annurev.ne.03.030180.001041. [DOI] [PubMed] [Google Scholar]
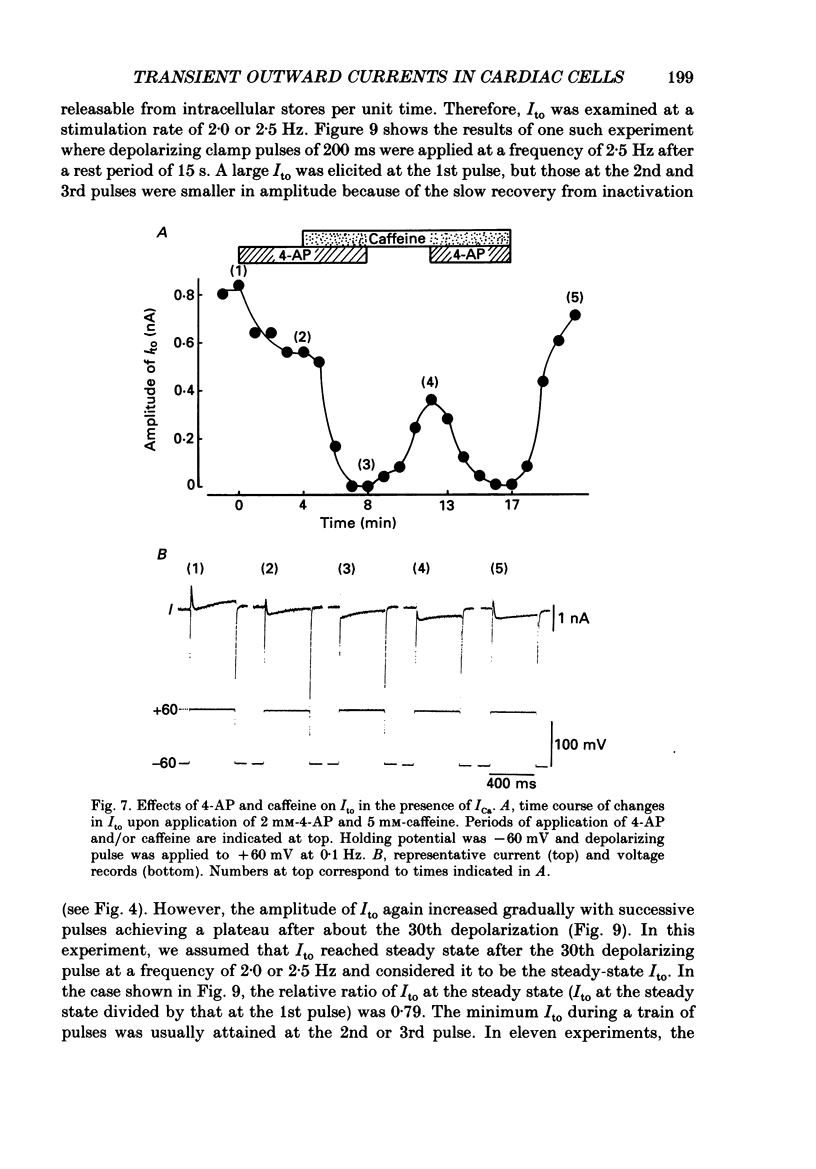
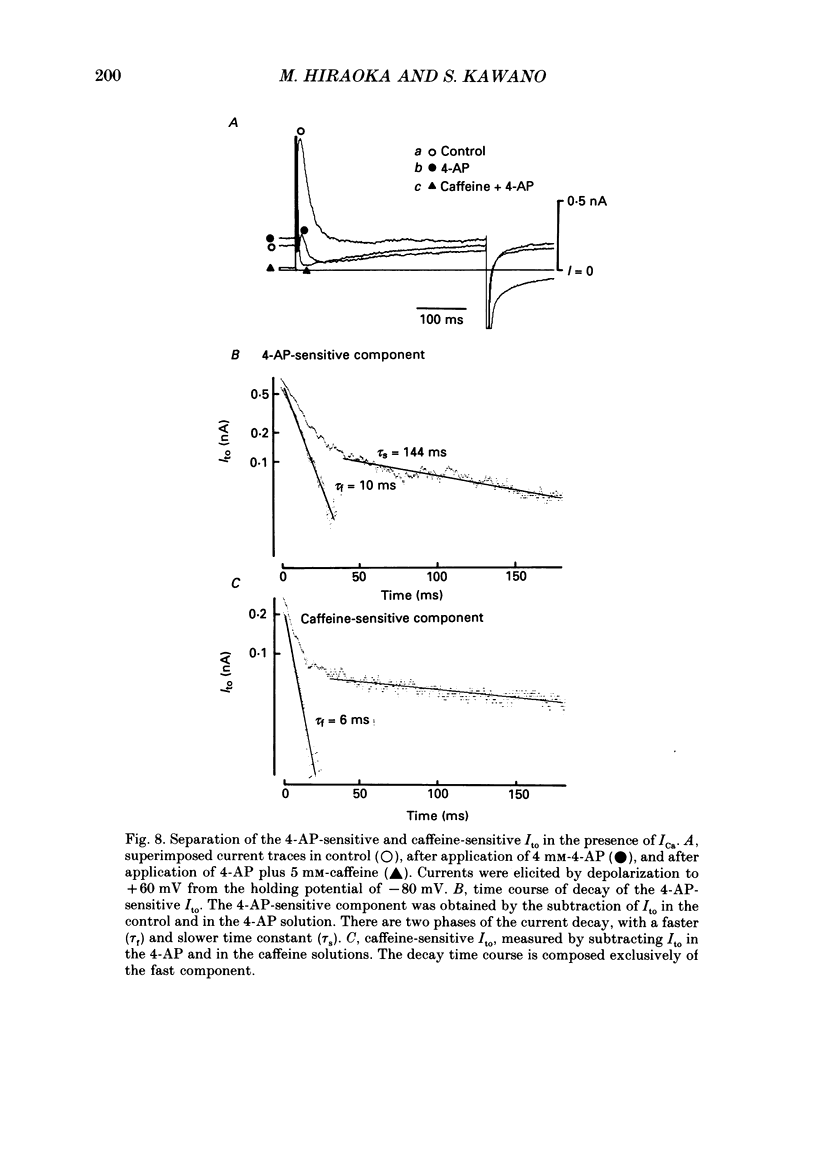
- Boyett M. R. A study of the effect of the rate of stimulation on the transient outward current in sheep cardiac Purkinje fibres. J Physiol. 1981;319:1–22. doi: 10.1113/jphysiol.1981.sp013888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R. Effect of rate-dependent changes in the transient outward current on the action potential in sheep Purkinje fibres. J Physiol. 1981;319:23–41. doi: 10.1113/jphysiol.1981.sp013889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A. Excitation-contraction coupling in cardiac muscle. Prog Biophys Mol Biol. 1979;35(1):1–52. doi: 10.1016/0079-6107(80)90002-4. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coraboeuf E., Carmeliet E. Existence of two transient outward currents in sheep cardiac Purkinje fibers. Pflugers Arch. 1982 Feb;392(4):352–359. doi: 10.1007/BF00581631. [DOI] [PubMed] [Google Scholar]
- Cukierman S., Paes de Carvalho A. P. Frequency-dependent excitability of "membrane" slow responses of Rabbit left atrial trabeculae in the presence of Ba2+ and high K+. J Gen Physiol. 1982 Jun;79(6):1017–1039. doi: 10.1085/jgp.79.6.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J., Peper K., Rüdel R., Trautwein W. The dynamic chloride component of membrane current in Purkinje fibers. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;295(3):197–212. doi: 10.1007/BF01844100. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Escande D., Coulombe A., Faivre J. F., Deroubaix E., Coraboeuf E. Two types of transient outward currents in adult human atrial cells. Am J Physiol. 1987 Jan;252(1 Pt 2):H142–H148. doi: 10.1152/ajpheart.1987.252.1.H142. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fozzard H. A., Hiraoka M. The positive dynamic current and its inactivation properties in cardiac Purkinje fibres. J Physiol. 1973 Nov;234(3):569–586. doi: 10.1113/jphysiol.1973.sp010361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W. R., van Ginneken A. C. A transient outward current in isolated cells from the crista terminalis of rabbit heart. J Physiol. 1985 Nov;368:243–264. doi: 10.1113/jphysiol.1985.sp015856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour R. F., Jr, Salata J. J., Davis J. R. Effects of 4-aminopyridine on rate-related depression of cardiac action potentials. Am J Physiol. 1986 Aug;251(2 Pt 2):H297–H306. doi: 10.1152/ajpheart.1986.251.2.H297. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hermann A., Hartung K. Ca2+ activated K+ conductance in molluscan neurones. Cell Calcium. 1983 Dec;4(5-6):387–405. doi: 10.1016/0143-4160(83)90016-7. [DOI] [PubMed] [Google Scholar]
- Hess P., Wier W. G. Excitation-contraction coupling in cardiac Purkinje fibers. Effects of caffeine on the intracellular [Ca2+] transient, membrane currents, and contraction. J Gen Physiol. 1984 Mar;83(3):417–433. doi: 10.1085/jgp.83.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka M., Hiraoka M. The role of the positive dynamic current on the action potential of cardiac Purkinje fibers. Jpn J Physiol. 1975;25(6):705–717. doi: 10.2170/jjphysiol.25.705. [DOI] [PubMed] [Google Scholar]
- Hiraoka M., Kawano S. Contribution of the transient outward current to the repolarization of rabbit ventricular cells. Jpn Heart J. 1986 Nov;27 (Suppl 1):77–82. [PubMed] [Google Scholar]
- Hiraoka M., Kawano S. Mechanism of increased amplitude and duration of the plateau with sudden shortening of diastolic intervals in rabbit ventricular cells. Circ Res. 1987 Jan;60(1):14–26. doi: 10.1161/01.res.60.1.14. [DOI] [PubMed] [Google Scholar]
- Iinuma H., Kato K. Mechanism of augmented premature responses in canine ventricular muscle. Circ Res. 1979 May;44(5):624–629. doi: 10.1161/01.res.44.5.624. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflugers Arch. 1982 Oct;395(1):30–41. doi: 10.1007/BF00584965. [DOI] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. Early outward current in rat single ventricular cells. Circ Res. 1984 Feb;54(2):157–162. doi: 10.1161/01.res.54.2.157. [DOI] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. 4-Aminopyridine and the early outward current of sheep cardiac Purkinje fibers. J Gen Physiol. 1979 Feb;73(2):139–157. doi: 10.1085/jgp.73.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. Effects of low-chloride solutions on action potentials of sheep cardiac Purkinje fibers. J Gen Physiol. 1977 Nov;70(5):635–660. doi: 10.1085/jgp.70.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. Influence of chloride, potassium, and tetraethylammonium on the early outward current of sheep cardiac Purkinje fibers. J Gen Physiol. 1979 Feb;73(2):117–138. doi: 10.1085/jgp.73.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukushkin N. I., Gainullin R. Z., Sosunov E. A. Transient outward current and rate dependence of action potential duration in rabbit cardiac ventricular muscle. Pflugers Arch. 1983 Oct;399(2):87–92. doi: 10.1007/BF00663902. [DOI] [PubMed] [Google Scholar]
- Mayer M. L. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol. 1985 Jul;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J., Morad M. A transient outward current related to calcium release and development of tension in elephant seal atrial fibres. J Physiol. 1984 Dec;357:267–292. doi: 10.1113/jphysiol.1984.sp015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Trautwein W. The potassium current underlying delayed rectification in cat ventricular muscle. J Physiol. 1978 Jan;274:217–246. doi: 10.1113/jphysiol.1978.sp012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Characteristics of the second inward current in cells isolated from rat ventricular muscle. Proc R Soc Lond B Biol Sci. 1983 Oct 22;219(1217):447–469. doi: 10.1098/rspb.1983.0084. [DOI] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Strontium, nifedipine and 4-aminopyridine modify the time course of the action potential in cells from rat ventricular muscle. Br J Pharmacol. 1984 Mar;81(3):551–556. doi: 10.1111/j.1476-5381.1984.tb10108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Fozzard H. A. Adrenergic modulation of the transient outward current in isolated canine Purkinje cells. Circ Res. 1988 Jan;62(1):162–172. doi: 10.1161/01.res.62.1.162. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Irisawa H. Transient outward current carried by potassium and sodium in quiescent atrioventricular node cells of rabbits. Circ Res. 1985 Jul;57(1):65–73. doi: 10.1161/01.res.57.1.65. [DOI] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper K., Trautwein W. A membrane current related to the plateau of the action potential of Purkinje fibers. Pflugers Arch. 1968;303(2):108–123. doi: 10.1007/BF00592629. [DOI] [PubMed] [Google Scholar]
- Schwarz W., Passow H. Ca2+-activated K+ channels in erythrocytes and excitable cells. Annu Rev Physiol. 1983;45:359–374. doi: 10.1146/annurev.ph.45.030183.002043. [DOI] [PubMed] [Google Scholar]
- Segal M., Rogawski M. A., Barker J. L. A transient potassium conductance regulates the excitability of cultured hippocampal and spinal neurons. J Neurosci. 1984 Feb;4(2):604–609. doi: 10.1523/JNEUROSCI.04-02-00604.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980 Feb;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko J. L., Ito K., Kenyon J. L. Ryanodine: a modifier of sarcoplasmic reticulum calcium release in striated muscle. Fed Proc. 1985 Dec;44(15):2984–2988. [PubMed] [Google Scholar]
- Tseng G. N., Robinson R. B., Hoffman B. F. Passive properties and membrane currents of canine ventricular myocytes. J Gen Physiol. 1987 Nov;90(5):671–701. doi: 10.1085/jgp.90.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Delbridge L. M., Bustamante J. O., McDonald T. F. Heterogeneity of the action potential in isolated rat ventricular myocytes and tissue. Circ Res. 1983 Mar;52(3):280–290. doi: 10.1161/01.res.52.3.280. [DOI] [PubMed] [Google Scholar]
- Yatani A., Imoto Y., Goto M. The effects of caffeine on the electrical properties of isolated, single rat ventricular cells. Jpn J Physiol. 1984;34(2):337–349. doi: 10.2170/jjphysiol.34.337. [DOI] [PubMed] [Google Scholar]


