Abstract
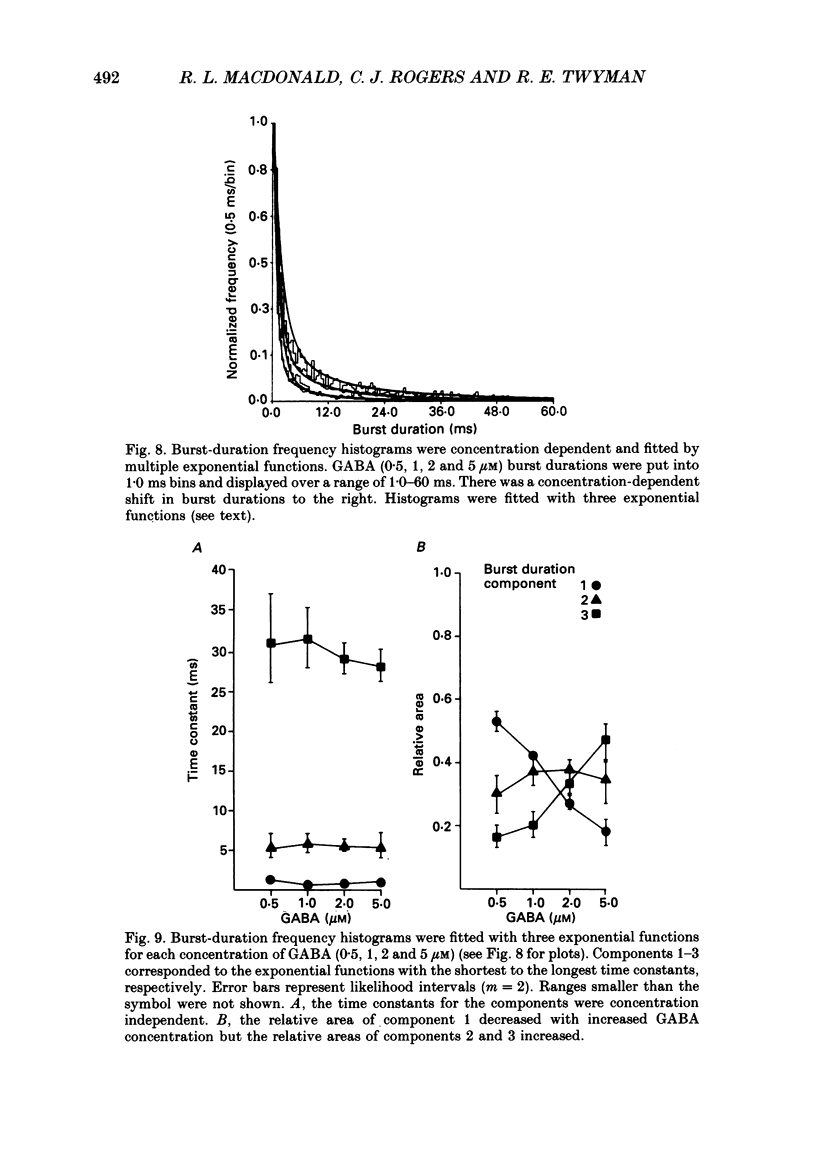
1. The kinetic properties of the main conductance state of gamma-aminobutyric acidA (GABA) receptor channels from somata of mouse spinal cord neurones in cell culture were investigated using patch clamp techniques. 2. Whole-cell GABA receptor currents increased in a concentration-dependent manner from 0.5 to 5 microM. 3. Single-channel currents were recorded with a main conductance state of 27.2 pS and a less frequent conductance state of 15.9 pS. The main conductance state opened singly and in bursts of several openings. 4. Mean open times of GABA receptor main conductance currents were increased and open-time frequency histograms were shifted to longer times as GABA concentration was increased from 0.5 to 5 microM. Three exponential functions were required to fit the histograms at all GABA concentrations, suggesting that the channel opened into at least three open states (O1, O2 and O3). The three functions had the same time constants (1.0 +/- 0.2, 3.7 +/- 0.4 and 11.3 +/- 0.5 ms; mean +/- S.D.) at each concentration. The increase in long open times with concentration was due to a shift in relative frequency of occurrence of openings from the shortest (O1) to the two longest (O2 and O3) open states. 5. Closed-time distributions of closures between main conductance state openings were fitted with multiple exponential functions, suggesting that the channel had several closed states. The two shortest time constants (0.24 +/- 0.03 and 2.0 +/- 0.3 ms) were concentration independent (0.5 to 5 microM). Three longer time constants decreased as concentration increased. 6. Bursts were defined as groups of openings surrounded by closures greater than a critical closed time (tc = 5 ms). Mean burst durations were increased and burst duration frequency histograms were shifted to longer times as GABA concentration was increased from 0.5 to 5 microM. Burst-duration frequency histograms were best fitted with three exponential functions. The time constants were concentration independent and were 1.0 +/- 0.2, 5.5 +/- 0.2 and 29.8 +/- 1.6 ms. The increase in burst duration with concentration was due to a relative shift from short duration bursts to longer duration bursts. 7. The shortest burst time constant was similar to the shortest open time constant suggesting that there was a population of single openings of short duration. The two longest burst time constants were longer than the two longest open time constants, suggesting that the bursts from the two longest burst components were composed of two or more openings.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



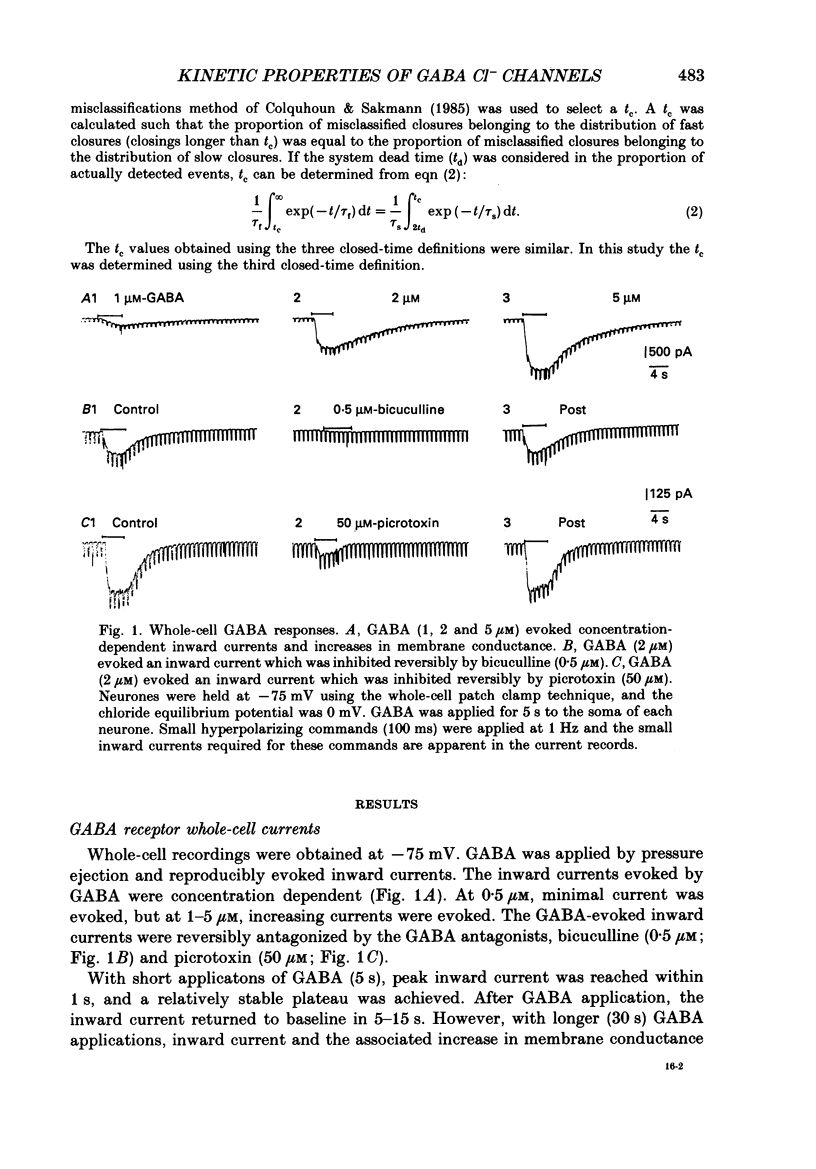

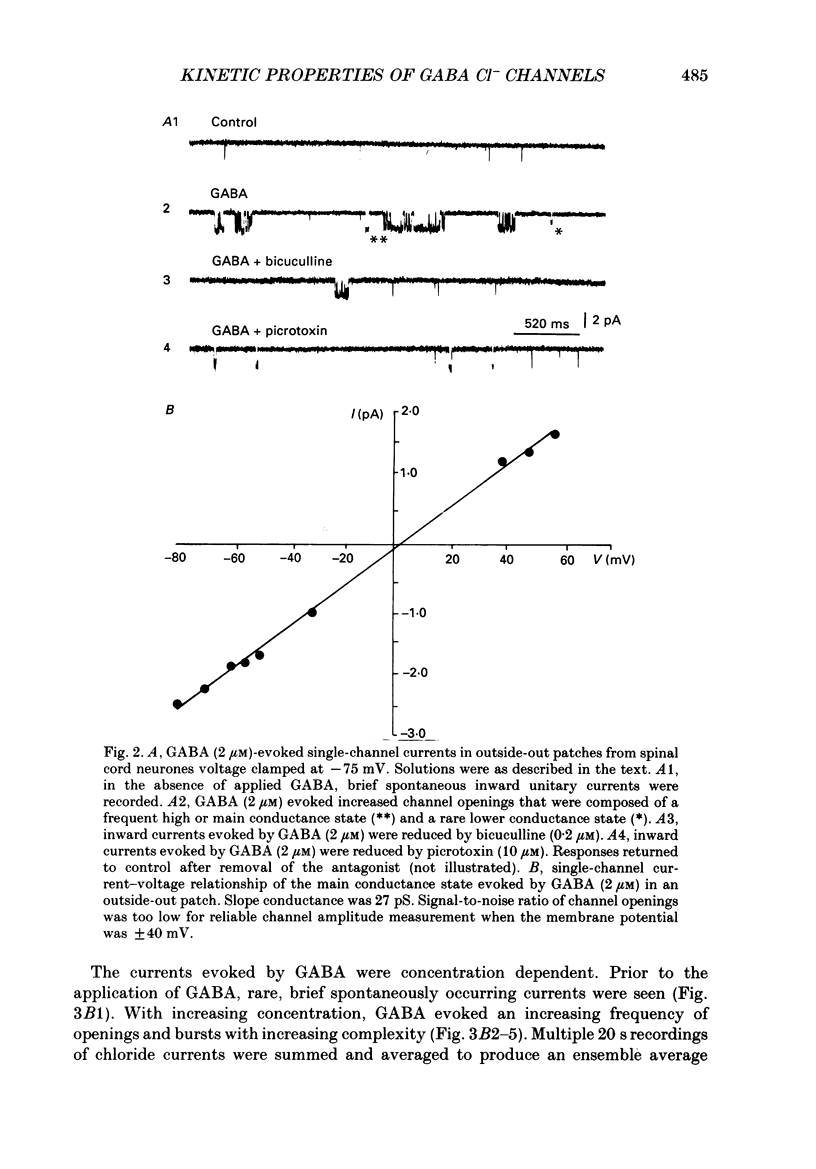
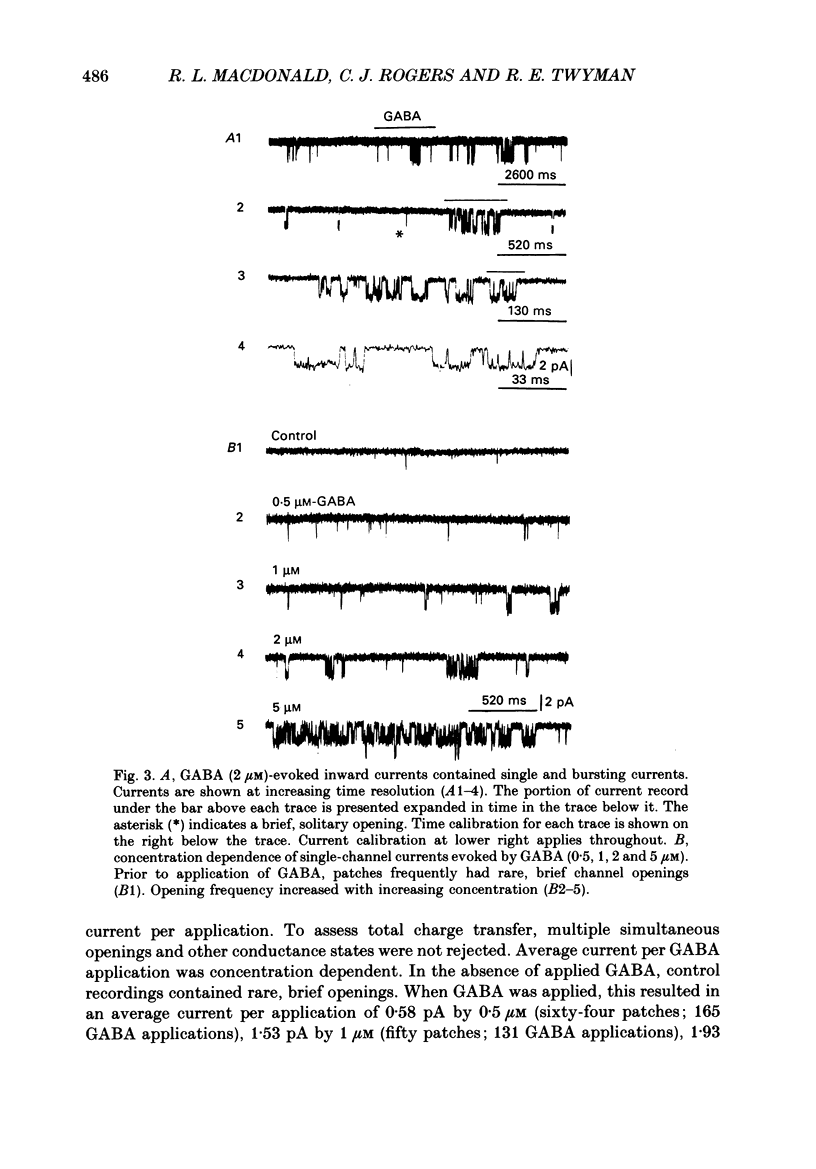
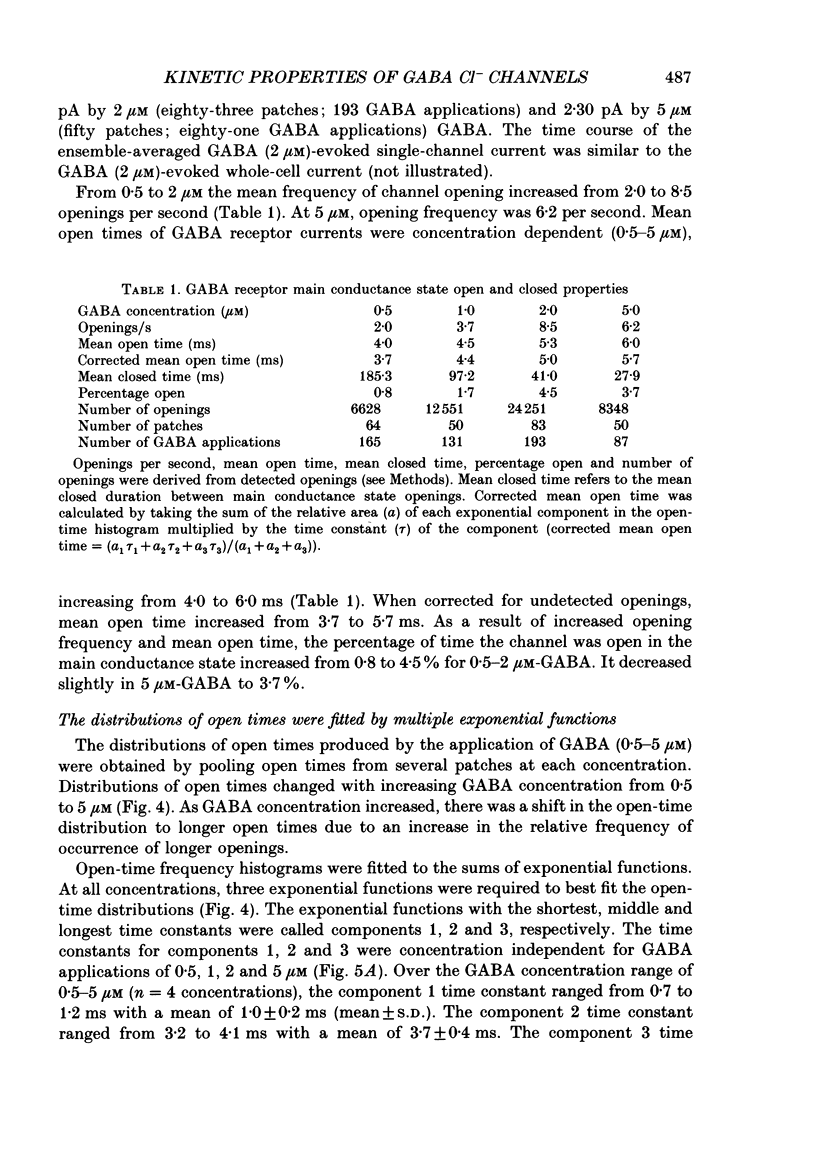
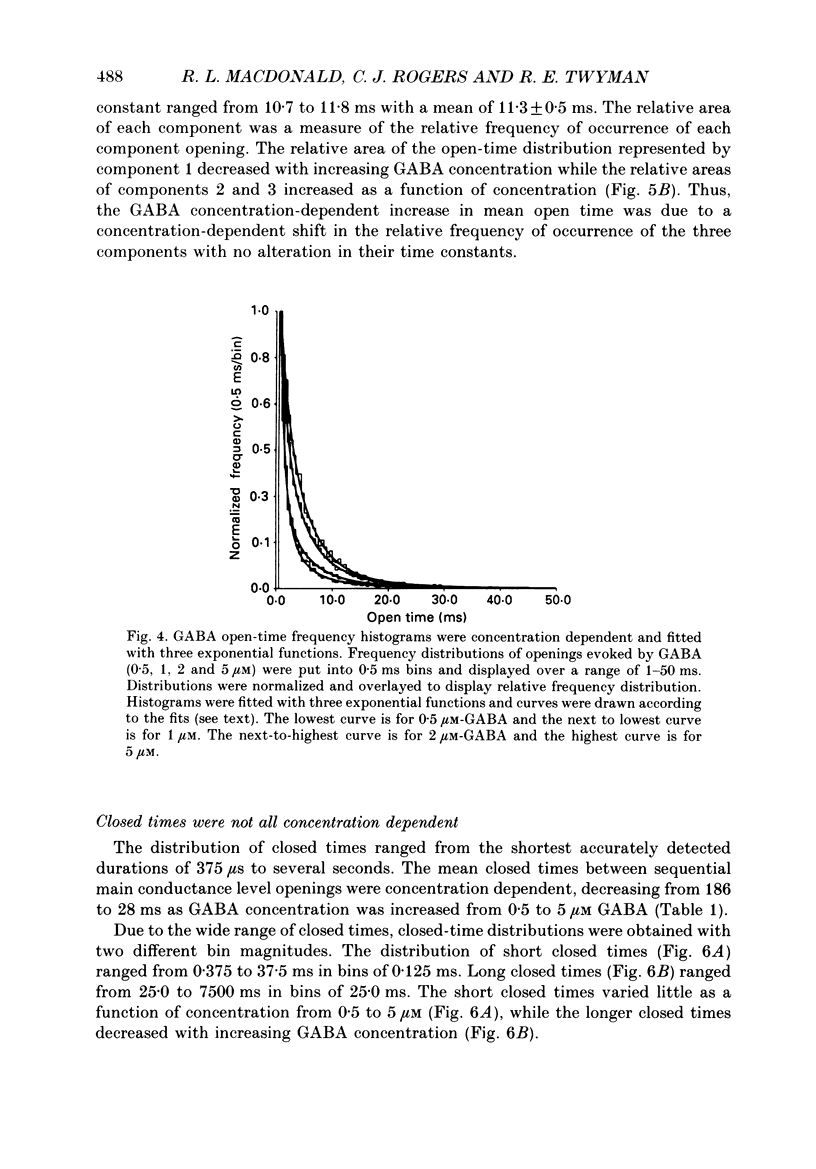
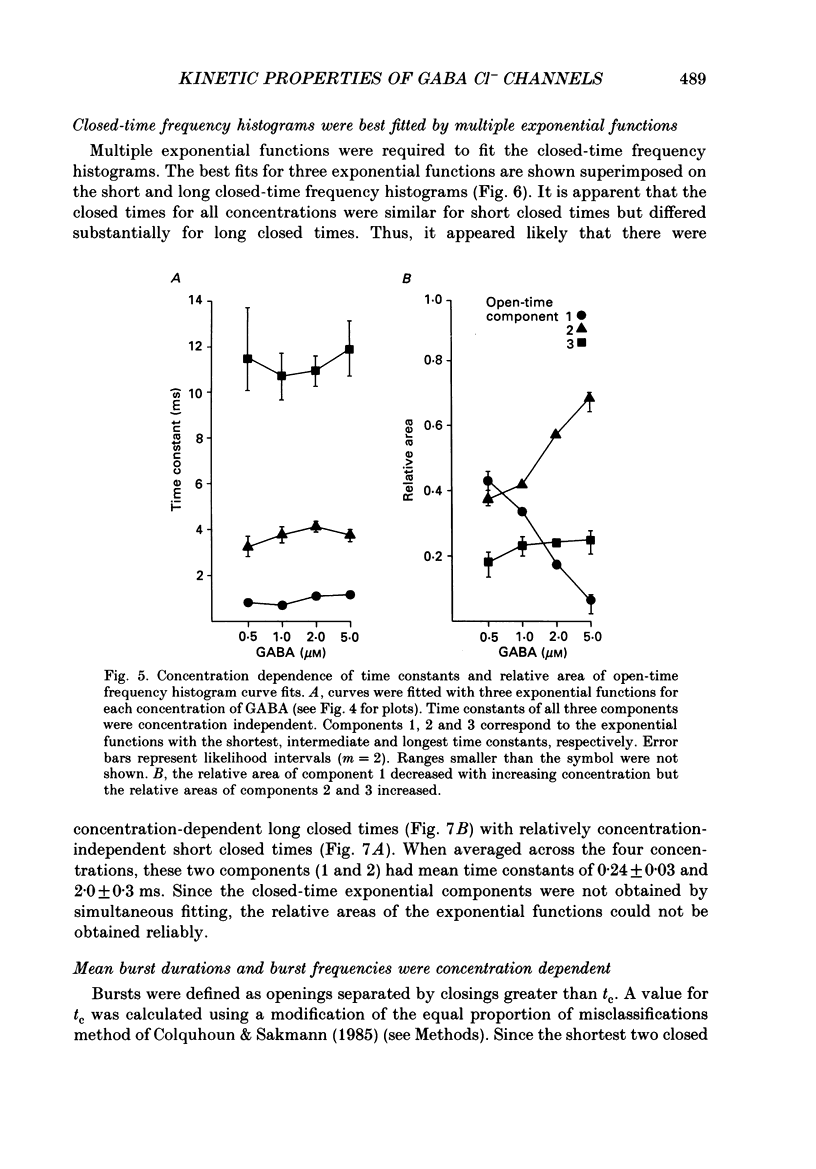







Selected References
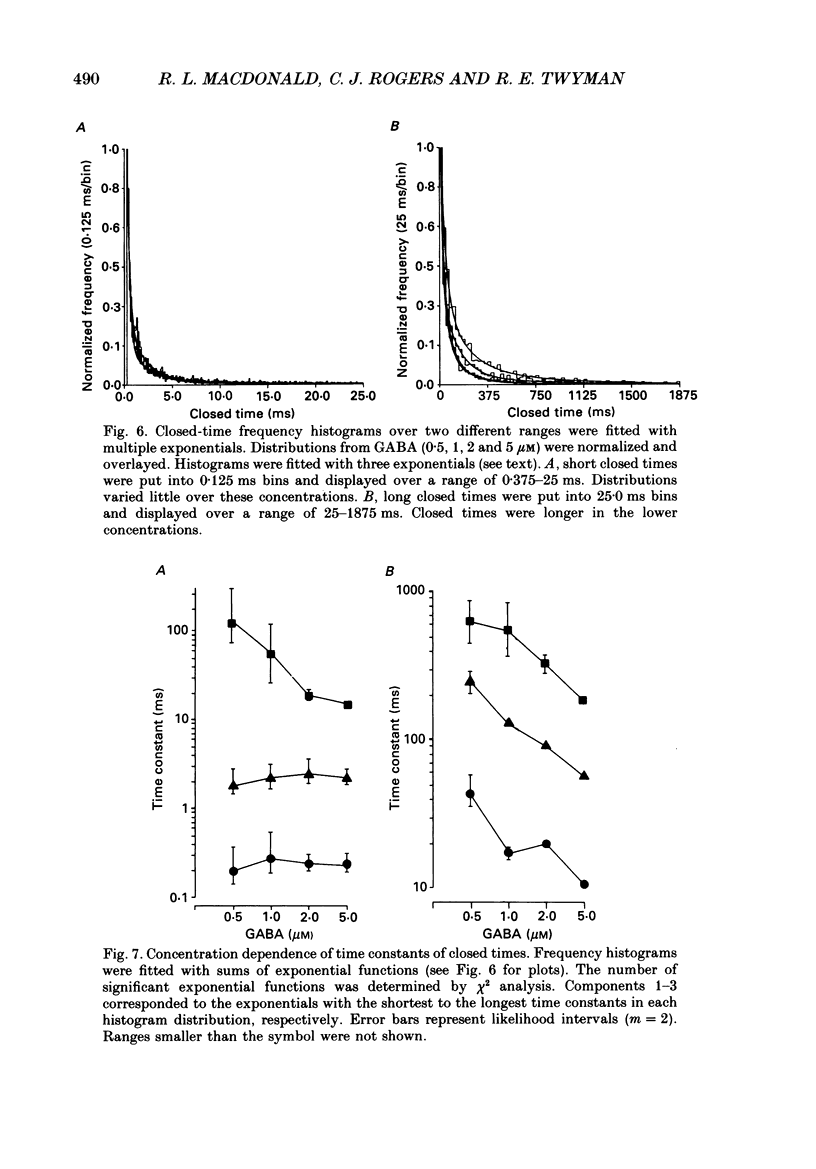
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Inomata N., Tokutomi N. Contribution of chloride shifts to the fade of gamma-aminobutyric acid-gated currents in frog dorsal root ganglion cells. J Physiol. 1987 Oct;391:219–234. doi: 10.1113/jphysiol.1987.sp016735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N., MacDonald J. F. Fluctuation analysis of neutral amino acid responses in cultured mouse spinal neurones. J Physiol. 1982 Jan;322:365–387. doi: 10.1113/jphysiol.1982.sp014042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Quantitative description of three modes of activity of fast chloride channels from rat skeletal muscle. J Physiol. 1986 Sep;378:141–174. doi: 10.1113/jphysiol.1986.sp016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Clapham D. E. gamma-Aminobutyric acid receptor channels in adrenal chromaffin cells: a patch-clamp study. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2168–2172. doi: 10.1073/pnas.82.7.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalotti S. O., Stephenson F. A., Barnard E. A. Separate subunits for agonist and benzodiazepine binding in the gamma-aminobutyric acidA receptor oligomer. J Biol Chem. 1986 Nov 15;261(32):15013–15016. [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Parker I. Single glutamate-activated channels recorded from locust muscle fibres with perfused patch-clamp electrodes. J Physiol. 1981 Dec;321:195–210. doi: 10.1113/jphysiol.1981.sp013979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J., Franke C. Single glutamate-gated synaptic channels at the crayfish neuromuscular junction. II. Dependence of channel open time on glutamate concentration. Pflugers Arch. 1987 Mar;408(3):307–314. doi: 10.1007/BF02181474. [DOI] [PubMed] [Google Scholar]
- Erickson J., Goldstein B., Holowka D., Baird B. The effect of receptor density on the forward rate constant for binding of ligands to cell surface receptors. Biophys J. 1987 Oct;52(4):657–662. doi: 10.1016/S0006-3495(87)83258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Bormann J., Sakmann B. Activation of multiple-conductance state chloride channels in spinal neurones by glycine and GABA. 1983 Oct 27-Nov 2Nature. 305(5937):805–808. doi: 10.1038/305805a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Jackson M. B. Dependence of acetylcholine receptor channel kinetics on agonist concentration in cultured mouse muscle fibres. J Physiol. 1988 Mar;397:555–583. doi: 10.1113/jphysiol.1988.sp017019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Lecar H., Mathers D. A., Barker J. L. Single channel currents activated by gamma-aminobutyric acid, muscimol, and (-)-pentobarbital in cultured mouse spinal neurons. J Neurosci. 1982 Jul;2(7):889–894. doi: 10.1523/JNEUROSCI.02-07-00889.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo F., Schuetze S. M. Kinetic differences between embryonic- and adult-type acetylcholine receptors in rat myotubes. J Physiol. 1988 Feb;396:267–296. doi: 10.1113/jphysiol.1988.sp016962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerry C. J., Ramsey R. L., Sansom M. S., Usherwood P. N. Glutamate receptor channel kinetics: the effect of glutamate concentration. Biophys J. 1988 Jan;53(1):39–52. doi: 10.1016/S0006-3495(88)83064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamalaki C., Stephenson F. A., Barnard E. A. The GABAA/benzodiazepine receptor is a heterotetramer of homologous alpha and beta subunits. EMBO J. 1987 Mar;6(3):561–565. doi: 10.1002/j.1460-2075.1987.tb04791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. J. gamma-Aminobutyric acid- and piperazine-activated single-channel currents from Ascaris suum body muscle. Br J Pharmacol. 1985 Feb;84(2):445–461. doi: 10.1111/j.1476-5381.1985.tb12929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers D. A., Barker J. L. (-)Pentobarbital opens ion channels of long duration in cultured mouse spinal neurons. Science. 1980 Jul 25;209(4455):507–509. doi: 10.1126/science.6248961. [DOI] [PubMed] [Google Scholar]
- Mathers D. A., Barker J. L. GABA- and glycine-induced Cl- channels in cultured mouse spinal neurons require the same energy to close. Brain Res. 1981 Nov 16;224(2):441–445. doi: 10.1016/0006-8993(81)90875-1. [DOI] [PubMed] [Google Scholar]
- Mathers D. A. Spontaneous and GABA-induced single channel currents in cultured murine spinal cord neurons. Can J Physiol Pharmacol. 1985 Oct;63(10):1228–1233. doi: 10.1139/y85-203. [DOI] [PubMed] [Google Scholar]
- McBurney R. N., Barker J. L. GABA-induced conductance fluctuations in cultured spinal neurones. Nature. 1978 Aug 10;274(5671):596–597. doi: 10.1038/274596a0. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- Nowak L. M., Young A. B., Macdonald R. L. GABA and bicuculline actions on mouse spinal cord and cortical neurons in cell culture. Brain Res. 1982 Jul 22;244(1):155–164. doi: 10.1016/0006-8993(82)90913-1. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Neale E., Henkart M., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. I. Morphology and intrinsic neuronal electrophysiologic properties. J Neurophysiol. 1977 Sep;40(5):1132–1150. doi: 10.1152/jn.1977.40.5.1132. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Hamill O. P., Bormann J. Patch-clamp measurements of elementary chloride currents activated by the putative inhibitory transmitter GABA and glycine in mammalian spinal neurons. J Neural Transm Suppl. 1983;18:83–95. [PubMed] [Google Scholar]
- Simmonds M. A. A site for the potentiation of GABA-mediated responses by benzodiazepines. Nature. 1980 Apr 10;284(5756):558–560. doi: 10.1038/284558a0. [DOI] [PubMed] [Google Scholar]


