Abstract
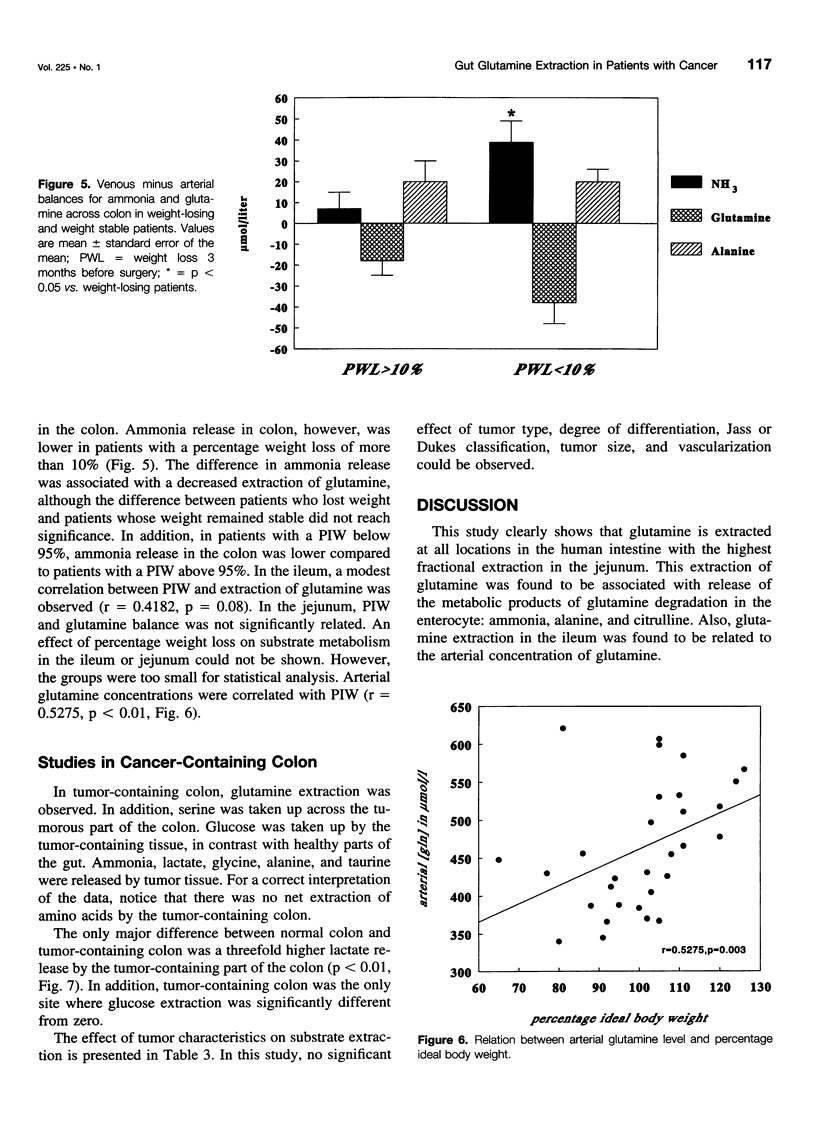
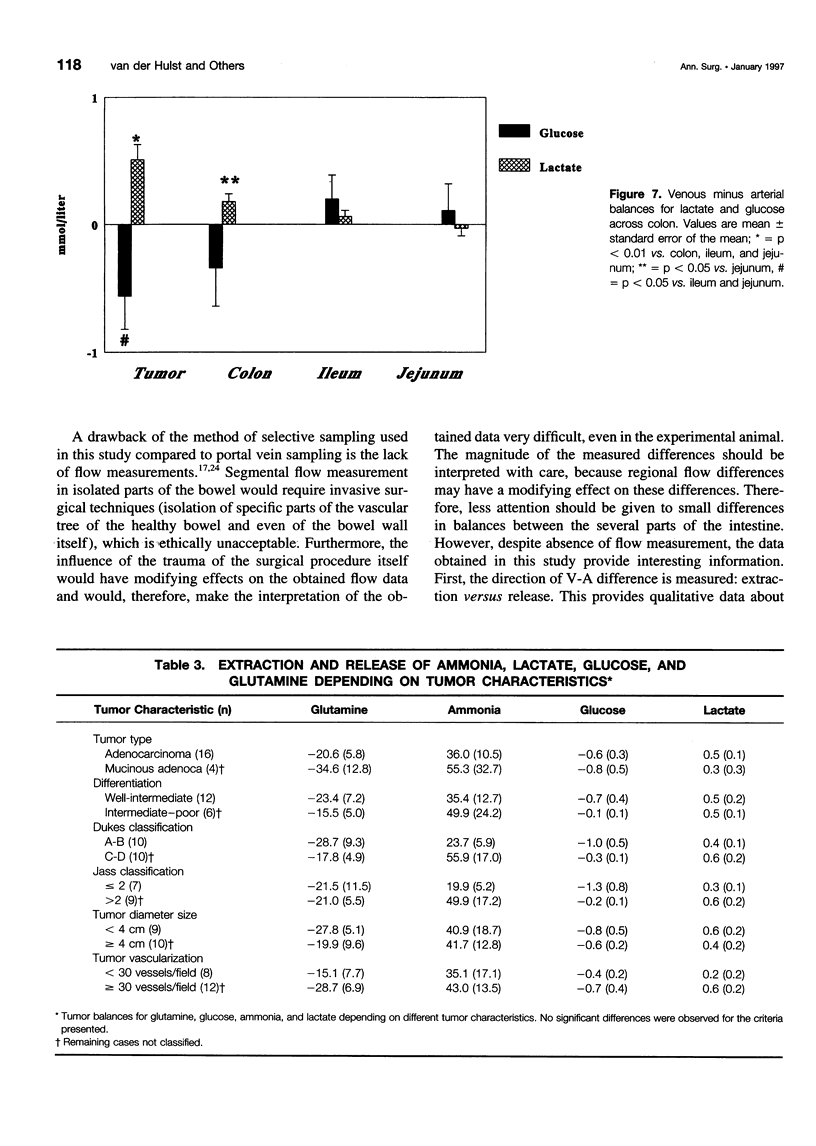
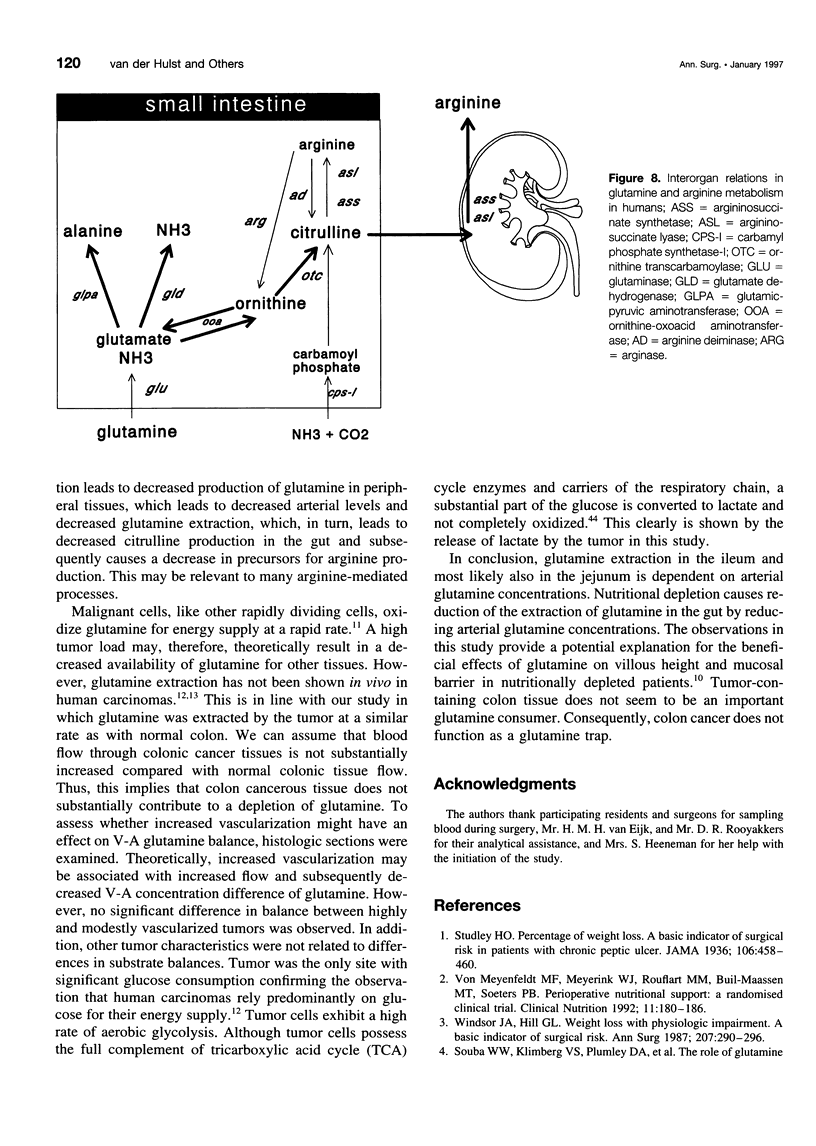
OBJECTIVE AND SUMMARY BACKGROUND DATA: Glutamine is an important fuel for the intestinal mucosa. However, glutamine pools may become depleted in the cancer-bearing host as a result of tumor consumption and diminished production due to nutritional depletion. As human data are lacking, the authors investigated glutamine extraction by different sites of the human intestine, including tumor and the potential relation with the degree of nutritional depletion. METHODS: Thirty-two patients with gastrointestinal malignancies were studied. Blood from an artery and veins draining jejunum, ileum, colon, or tumor were sampled. Depletion was estimated by the percentage ideal body weight. RESULTS: Fractional glutamine extraction rate in the jejunum was 24%, three times higher than in ileum and colon. Percentage ideal body weight correlated with arterial glutamine levels (r = 0.5275, p = 0.003). In addition, arterial glutamine concentrations were correlated with extraction in the ileum (r = -0.8411, p < 0.001). Colon-containing tumor did not extract more glutamine than did nontumor-containing colon. CONCLUSIONS: Glutamine is a quantitatively more important substrate for the proximal intestine than for the distal gut. Nutritional depletion results in decreased arterial glutamine concentration, which in turn results in diminished extraction. Colon cancer does not function as a glutamine trap and does not contribute to glutamine depletion.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brosnan J. T. The 1986 Borden award lecture. The role of the kidney in amino acid metabolism and nutrition. Can J Physiol Pharmacol. 1987 Dec;65(12):2355–2362. doi: 10.1139/y87-373. [DOI] [PubMed] [Google Scholar]
- Dejong C. H., Deutz N. E., Soeters P. B. Metabolic adaptation of the kidney to hyperammonemia during chronic liver insufficiency in the rat. Hepatology. 1993 Oct;18(4):890–902. doi: 10.1002/hep.1840180422. [DOI] [PubMed] [Google Scholar]
- Dejong C. H., Kampman M. T., Deutz N. E., Soeters P. B. Altered glutamine metabolism in rat portal drained viscera and hindquarter during hyperammonemia. Gastroenterology. 1992 Mar;102(3):936–948. doi: 10.1016/0016-5085(92)90180-7. [DOI] [PubMed] [Google Scholar]
- Deutz N. E., Reijven P. L., Athanasas G., Soeters P. B. Post-operative changes in hepatic, intestinal, splenic and muscle fluxes of amino acids and ammonia in pigs. Clin Sci (Lond) 1992 Nov;83(5):607–614. doi: 10.1042/cs0830607. [DOI] [PubMed] [Google Scholar]
- Eriksson L. S., Olsson M., Björkman O. Splanchnic metabolism of amino acids in healthy subjects: effect of 60 hours of fasting. Metabolism. 1988 Dec;37(12):1159–1162. doi: 10.1016/0026-0495(88)90194-1. [DOI] [PubMed] [Google Scholar]
- Featherston W. R., Rogers Q. R., Freedland R. A. Relative importance of kidney and liver in synthesis of arginine by the rat. Am J Physiol. 1973 Jan;224(1):127–129. doi: 10.1152/ajplegacy.1973.224.1.127. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J., Karl I., Cerasi E., Luft R., Kipnis D. M. Glutamine and glutamate metabolism in normal and diabetic subjects. Diabetes. 1973 Aug;22(8):573–576. doi: 10.2337/diab.22.8.573. [DOI] [PubMed] [Google Scholar]
- Fleming S. E., Fitch M. D., DeVries S., Liu M. L., Kight C. Nutrient utilization by cells isolated from rat jejunum, cecum and colon. J Nutr. 1991 Jun;121(6):869–878. doi: 10.1093/jn/121.6.869. [DOI] [PubMed] [Google Scholar]
- Hagmüller E., Kollmar H. B., Günther H. J., Holm E., Trede M. Protein metabolism in human colon carcinomas: in vivo investigations using a modified tracer technique with L-[1-13C]leucine. Cancer Res. 1995 Mar 1;55(5):1160–1167. [PubMed] [Google Scholar]
- Hammarqvist F., Wernerman J., Ali R., von der Decken A., Vinnars E. Addition of glutamine to total parenteral nutrition after elective abdominal surgery spares free glutamine in muscle, counteracts the fall in muscle protein synthesis, and improves nitrogen balance. Ann Surg. 1989 Apr;209(4):455–461. doi: 10.1097/00000658-198904000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm E., Hagmüller E., Staedt U., Schlickeiser G., Günther H. J., Leweling H., Tokus M., Kollmar H. B. Substrate balances across colonic carcinomas in humans. Cancer Res. 1995 Mar 15;55(6):1373–1378. [PubMed] [Google Scholar]
- Hurwitz R., Kretchmer N. Development of arginine-synthesizing enzymes in mouse intestine. Am J Physiol. 1986 Jul;251(1 Pt 1):G103–G110. doi: 10.1152/ajpgi.1986.251.1.G103. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Espat N. J., Frohnapple D. J., Epstein H., Copeland E. M., Souba W. W. Effect of total parenteral nutrition on amino acid and glucose transport by the human small intestine. Ann Surg. 1993 Jun;217(6):604–614. doi: 10.1097/00000658-199306000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochs H., Roth E., Gasic S., Hübl W., Morse E. L., Adibi S. A. Splanchnic, renal, and muscle clearance of alanylglutamine in man and organ fluxes of alanine and glutamine when infused in free and peptide forms. Metabolism. 1990 Aug;39(8):833–836. doi: 10.1016/0026-0495(90)90128-y. [DOI] [PubMed] [Google Scholar]
- Marliss E. B., Aoki T. T., Pozefsky T., Most A. S., Cahill G. F., Jr Muscle and splanchnic glutmine and glutamate metabolism in postabsorptive andstarved man. J Clin Invest. 1971 Apr;50(4):814–817. doi: 10.1172/JCI106552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnena O. J., Moore F. A., Moore E. E., Jones T. N., Parsons P. Selective uptake of glutamine in the gastrointestinal tract: confirmation in a human study. Br J Surg. 1991 Apr;78(4):480–482. doi: 10.1002/bjs.1800780429. [DOI] [PubMed] [Google Scholar]
- NEPTUNE E. M., Jr RESPIRATION AND OXIDATION OF VARIOUS SUBSTRATES BY ILEUM IN VITRO. Am J Physiol. 1965 Aug;209:329–332. doi: 10.1152/ajplegacy.1965.209.2.329. [DOI] [PubMed] [Google Scholar]
- Roediger W. E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980 Sep;21(9):793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheppach W., Loges C., Bartram P., Christl S. U., Richter F., Dusel G., Stehle P., Fuerst P., Kasper H. Effect of free glutamine and alanyl-glutamine dipeptide on mucosal proliferation of the human ileum and colon. Gastroenterology. 1994 Aug;107(2):429–434. doi: 10.1016/0016-5085(94)90168-6. [DOI] [PubMed] [Google Scholar]
- Souba W. W. Glutamine and cancer. Ann Surg. 1993 Dec;218(6):715–728. doi: 10.1097/00000658-199312000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souba W. W., Herskowitz K., Klimberg V. S., Salloum R. M., Plumley D. A., Flynn T. C., Copeland E. M., 3rd The effects of sepsis and endotoxemia on gut glutamine metabolism. Ann Surg. 1990 May;211(5):543–551. doi: 10.1097/00000658-199005000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souba W. W., Herskowitz K., Salloum R. M., Chen M. K., Austgen T. R. Gut glutamine metabolism. JPEN J Parenter Enteral Nutr. 1990 Jul-Aug;14(4 Suppl):45S–50S. doi: 10.1177/014860719001400403. [DOI] [PubMed] [Google Scholar]
- Souba W. W., Klimberg V. S., Plumley D. A., Salloum R. M., Flynn T. C., Bland K. I., Copeland E. M., 3rd The role of glutamine in maintaining a healthy gut and supporting the metabolic response to injury and infection. J Surg Res. 1990 Apr;48(4):383–391. doi: 10.1016/0022-4804(90)90080-l. [DOI] [PubMed] [Google Scholar]
- Stehle P., Zander J., Mertes N., Albers S., Puchstein C., Lawin P., Fürst P. Effect of parenteral glutamine peptide supplements on muscle glutamine loss and nitrogen balance after major surgery. Lancet. 1989 Feb 4;1(8632):231–233. doi: 10.1016/s0140-6736(89)91254-3. [DOI] [PubMed] [Google Scholar]
- Tamada H., Nezu R., Imamura I., Matsuo Y., Takagi Y., Kamata S., Okada A. The dipeptide alanyl-glutamine prevents intestinal mucosal atrophy in parenterally fed rats. JPEN J Parenter Enteral Nutr. 1992 Mar-Apr;16(2):110–116. doi: 10.1177/0148607192016002110. [DOI] [PubMed] [Google Scholar]
- Van Der Hulst R. R., Deutz N. E., Von Meyenfeldt M. F., Elbers J. M., Stockbrügger R. W., Soeters P. B. Decrease of mucosal glutamine concentration in the nutritionally depleted patient. Clin Nutr. 1994 Aug;13(4):228–233. doi: 10.1016/0261-5614(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Vermeulen P. B., Verhoeven D., Fierens H., Hubens G., Goovaerts G., Van Marck E., De Bruijn E. A., Van Oosterom A. T., Dirix L. Y. Microvessel quantification in primary colorectal carcinoma: an immunohistochemical study. Br J Cancer. 1995 Feb;71(2):340–343. doi: 10.1038/bjc.1995.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Meyenfeldt M. F., Meijerink W. J., Rouflart M. M., Builmaassen M. T., Soeters P. B. Perioperative nutritional support: a randomised clinical trial. Clin Nutr. 1992 Aug;11(4):180–186. doi: 10.1016/0261-5614(92)90026-m. [DOI] [PubMed] [Google Scholar]
- Wilmore D. W., Smith R. J., O'Dwyer S. T., Jacobs D. O., Ziegler T. R., Wang X. D. The gut: a central organ after surgical stress. Surgery. 1988 Nov;104(5):917–923. [PubMed] [Google Scholar]
- Windmueller H. G. Glutamine utilization by the small intestine. Adv Enzymol Relat Areas Mol Biol. 1982;53:201–237. doi: 10.1002/9780470122983.ch6. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Respiratory fuels and nitrogen metabolism in vivo in small intestine of fed rats. Quantitative importance of glutamine, glutamate, and aspartate. J Biol Chem. 1980 Jan 10;255(1):107–112. [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem. 1974 Aug 25;249(16):5070–5079. [PubMed] [Google Scholar]
- Windsor J. A., Hill G. L. Weight loss with physiologic impairment. A basic indicator of surgical risk. Ann Surg. 1988 Mar;207(3):290–296. doi: 10.1097/00000658-198803000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler T. R., Young L. S., Benfell K., Scheltinga M., Hortos K., Bye R., Morrow F. D., Jacobs D. O., Smith R. J., Antin J. H. Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition after bone marrow transplantation. A randomized, double-blind, controlled study. Ann Intern Med. 1992 May 15;116(10):821–828. doi: 10.7326/0003-4819-116-10-821. [DOI] [PubMed] [Google Scholar]
- van Berlo C. L., van den Bogaard A. E., Bost M. C., Soeters P. B. A technique to study splanchnic metabolism in the unrestrained conscious pig. Lab Anim Sci. 1988 Aug;38(4):463–466. [PubMed] [Google Scholar]
- van Eijk H. M., Rooyakkers D. R., Deutz N. E. Rapid routine determination of amino acids in plasma by high-performance liquid chromatography with a 2-3 microns Spherisorb ODS II column. J Chromatogr. 1993 Oct 22;620(1):143–148. doi: 10.1016/0378-4347(93)80062-9. [DOI] [PubMed] [Google Scholar]
- van der Hulst R. R., van Kreel B. K., von Meyenfeldt M. F., Brummer R. J., Arends J. W., Deutz N. E., Soeters P. B. Glutamine and the preservation of gut integrity. Lancet. 1993 May 29;341(8857):1363–1365. doi: 10.1016/0140-6736(93)90939-e. [DOI] [PubMed] [Google Scholar]


