Abstract
Background
Hearing loss adversely impacts language development, acquisition, and the social and cognitive maturation of affected children. The hearing loss etiology mainly includes genetic factors and environmental factors, of which the former account for about 50–60%.
Objective
This study aimed to investigate the genetic basis of autosomal recessive non-syndromic hearing loss (NSHL) by identifying and characterizing novel variants in the CDH23 gene. Furthermore, it seeks to determine the pathogenic potential of the noncanonical splice site variant c.2398-6G > A.
Methods
Comprehensive clinical evaluation and whole-exome sequencing (WES) were performed on the girl. The WES analysis revealed two novel variants in the CDH23 gene, associated with nonsyndromic deafness 12 (DFNB12). To further explore the pathogenicity of these variants, functional studies involving in vivo splicing analysis were performed on the novel noncanonical splice site variant, c.2398-6G > A, which was initially classified as a variant of uncertain significance (VUS).
Results
Whole-exome sequencing of the patient identified two compound heterozygous variants in CDH23: c.2398-6G > A, a noncanonical splice site variant, and c.6068C > A (p. Ser2023Ter), a nonsense mutation. In vitro splicing assays demonstrated that c.2398-6G > A caused aberrant splicing, leading to a frameshift (p. Val800Alafs*6) and the production of a truncated protein, as confirmed by structural protein analysis. The study revealed novel mutations as likely pathogenic, linking both variants to autosomal recessive NSHL.
Conclusions
Our analyses revealed novel compound heterozygous mutations in CDH23 associated with autosomal recessive NSHL, thereby expanding the mutational landscape of CDH23-related hearing loss and increasing knowledge about the CDH23 splice site variants.
Keywords: Nonsyndromic hearing loss, CDH23, Autosomal recessive, Whole-exome sequencing, Noncanonical splice site variant
Introduction
Hearing loss is one of the most common sensory deficit diseases, which can occur in people of all ages, leading to severe speech communication disorders and affecting the patients, quality of life. Deafness exhibits a high prevalence, with a complex and multifactorial etiology. According to the World Hearing Report released by the WHO in 2021, there are as many as 1.5 billion people with deafness in the world at present, and it is expected to rise to 2.5 billion by 2050 (Chadha et al. 2021). The incidence of deafness in infants worldwide is about 2–6% (Olusanya 2012).
The hearing loss etiology mainly includes genetic factors and environmental factors, of which the former account for about 50–60% (Yuan et al. 2020). Congenital hearing loss stems from genetic causes that are split into two categories: syndromic, involving other medical conditions, and non-syndromic, where hearing loss is the sole manifestation. Non-syndromic hearing loss (NSHL) constitutes 75% of all inherited hearing impairments. Of these cases, about 80% are caused by autosomal recessive genes, 15–20% by autosomal dominant genes, and 1–2% by either mitochondrial or X-linked mutations (Alford et al. 2014). Syndromic hearing loss is related to other symptoms, comprises the remaining 30% of genetic hearing loss cases (Smith et al. 2005; Lammens et al. 2013). The rapid development of genetic testing technology, especially the next-generation sequencing, has promoted the exploration of the pathogenesis of hereditary deafness. A growing number of deafness genes have been identified, has been clear as many as 224 kinds of deafness genes. The common pathogenic factors in China are GJB2, SLC26A4, and CDH23 (Du et al. 2016).
Recessive mutations of the CDH23 gene (OMIM: 605,516) are responsible for both nonsyndromic deafness 12 (DFNB12, OMIM: 601,386) and Usher syndrome type 1D (USH1D, OMIM: 601,067) (Bolz et al. 2001; Bork et al. 2001a; Astuto et al. 2002). DFNB12 is characterized by prelingual-onset sensorineural NSHL, without the impairment of visual or vestibular functions. In contrast, individuals with USH1D exhibit severe phenotype, encompassing congenital severe to profound deafness, variable vestibular areflexia, and progressive adolescent-onset vision loss due to retinitis pigmentosa (RP) (Bork et al. 2002; Oshima et al. 2008; Friedman et al. 2011). The encoded protein cadherin 23 (CDH23) is a member of the cadherin super family which constitutes calcium-dependent cell–cell adhesion glycoproteins and is known to be expressed in both the inner and outer hair cells in the cochlea. Encoded protein cadherin 23 comprises the “Tip Link” structure of the stereocilia important for hair cell function. CDH23 has essential roles in establishing and maintaining the proper organization of the stereocilia bundle of hair cells in the cochlea and vestibule during late embryonic and early postnatal development (Ramzan et al. 2020). Notably, a number of CDH23 mutations have been described to date, revealing an interesting genotype–phenotype correlation shown in Table 1.
Table 1.
Splicing mutations described in the CDH23 gene, with their nucleotide position and associated phenotype (from the Human Gene Mutation Database)
| Mutation | HGMD access ID | Mutation type | Disease |
|---|---|---|---|
| 2177-2A > G | CS1110166 | IVS19 as A-G -2 | Usher syndrome 1 |
| 2176+1G > C | CS1610394 | IVS19 ds G-C + 1 | Deafness, non-syndromic, autosomal recessive |
| 2290-1G > A | CS2329085 | IVS20 as G-A -1 | Hearing loss, non-syndromic |
| 2289+1G > A | CS021740 | IVS20 ds G-A + 1 | Usher syndrome 1 |
| 2289+6T > G | CS186197 | IVS20 ds T-G + 6 | Hearing loss, non-syndromic |
| 2398-1G > T | CS1613921 | IVS21 as G-T -1 | Retinal disease |
| 2587+1G > T | CS088228 | IVS22 ds G-T + 1 | Usher syndrome 1 |
| 6030T > G | CM2257689 | Phe2010Leu | Hereditary diffuse gastric cancer |
| 6049G > A | CM077923 | Gly2017Ser | Usher syndrome 1 |
| 6083A > C | CM190105 | Asp2028Ala | Hearing loss, non-syndromic |
| 6085C > T | CM074086 | Arg2029Trp | Hearing loss, non-syndromic |
| 6098C > A | CM226757 | Ser2033Term | Hearing loss |
| 6133G > A | CM010181 | Asp2045Asn | Non-syndromic autosomal recessive deafness |
DFNB12 is characterized by prelingual-onset sensorineural hearing loss (SNHL) with no vestibular or visual involvement, typically showing a stable, non-progressive course (Zhong 2013). In contrast, USH1D, a more severe form, is associated with congenital profound hearing loss, vestibular areflexia, and progressive vision loss due to retinitis pigmentosa (RP), which begins in adolescence (Tb et al. 2011). This spectrum of phenotypes is largely influenced by the specific mutations in the CDH23 gene, which exhibit a genotype–phenotype correlation that can provide valuable insights into diagnosis and prognosis. In particular, compound heterozygous mutations in CDH23 have been increasingly recognized as an important cause of DFNB12 and USH1D, and their functional consequences have been studied extensively in recent years (Bork et al. 2001b). Advances in molecular genetic testing and the ability to detect such mutations using techniques such as whole-exome sequencing (WES) and Sanger sequencing have enabled more accurate diagnosis and better clinical management of affected families.
In the current study, we describe a Chinese family in which the youngest daughter had profound high-frequency progressive NSHL, without any vestibular or ocular involvement. Detailed clinical and molecular genetic analyses were performed through WES and Sanger sequencing. We identified two novel candidate variants in CDH23, one of which is intronic variation in the junctional region (c.2398-6G > A), was evaluated “variant of uncertain significance (VUS)” based on the latest ACMG guidelines (Richards et al. 2015). To further evaluate this novel VUS variant, we performed in vivo splicing analysis. We confirmed the compound heterozygosis in CDH23 as the cause of NSHL in the girl with late onset autosomal recessive hearing loss. The implications of our discovery are valuable in the clinical diagnosis, prognosis, and treatment of patients with CDH23 pathogenic variants.
Material and methods
Patients and clinical data
An 8-year-old Chinese girl presented with hearing loss after suspicion based on regression of speech. She is the second child born to healthy, non-consanguineous parents and has a healthy elder sister. No significant medical history was noted during the prenatal, delivery, and postpartum periods. The patient successfully passed the newborn hearing screening, with no abnormalities detected at that time. Her early language milestones were within the expected range. She began babbling at 8–9 months and spoke her first words by 12 months. By the age of 2 years, she was capable of speaking longer words. However, a gradual regression in her language skills was observed starting at the age of 3 years. Currently, her verbal communication is predominantly limited to the use of repetitive and simple words. She is using a hearing aid, which provides some improvement in her auditory capabilities.
Clinical evaluation of subject
The girl was thoroughly examined in the department of paediatrics and otolaryngology at Ganzhou Maternal and Child Health Hospital. Detailed medical histories and physical examinations, such as computerized tomography (CT) scans of the temporal bonehead, magnetic resonance (MR) labyrinthography and eye fundus were carried out to exclude any possible environmental causes or syndromic forms of hearing loss. Audiological evaluation included tympanometry, distortion product otoacoustic emission (DPOAE), click-evoked air-conduction auditory brain-stem response (AC-ABR), click-evoked bone-conduction auditory brain-stem response (BC-ABR), pure-tone audiometry (PTA). The hearing levels were determined by the thresholds of AC-ABR, NB CE-chirp ASSR, or PTA. Further, according to the World Report on Hearing in 2021, the grades of hearing loss were classified as mild (20 to < 35 dB HL), moderate (35 to < 50 dB HL), moderately severe (50 to < 65 dB HL), severe (65 to < 80 dB HL), or profound (80 to < 95 dB HL), complete or total (> 95 dB HL) by the average threshold of PTA air conduction in 500 Hz, 1000 Hz, 2000 Hz and 4000 Hz. In order to estimate the progress of her hearing levels, we evaluated her hearing in her 7 and 7.5 years old respectively by DPOAE, AC-ABR and BC-ABR. PTA was conducted when she was 8 years old.
Sample collection
This research was approved by the Ethics Committee of the Ganzhou Maternal and Child Health Hospital. Peripheral venous blood samples were taken from the girl, her parents and her elder sister following the acquisition of informed consent from all participants. RNA was extracted using the PAXgene Blood RNA Kit (PreAnalytiX, Hombrechtikon, Switzerland). In addition, DNA was extracted from the peripheral blood using the QIAamp DNA Mini Kit for blood (Qiagen, Hilden, Germany). Trio-based WES was performed by KingMed Diagnostics (Guangzhou, China), including exome library preparation, sequencing, and data analysis. Variant screening was based on clinical phenotypes of the affected subjects, population database (dbSNP, 1,000 Genome, ExAC), disease database (OMIM, HGMD, Clinvar), and biological information prediction tools (SIFT, Polyphen2, Mutation Taster and Splice AI). Two novel variants of CDH23 were identified in the girl, one is a splicing variant (CDH23: c.2398-6G > A) inherited her mother, and the another is a nonsense variant (CDH23: c.6068C > A), inherited from her father. The two CDH23 variants were confirmed by Sanger sequencing. To identify the potential impact of the c.2398-6G > A noncanonical splice variant of CDH23, the RDDCSC online in silico splice site prediction software (https://rddc.tsinghua-gd.org/search-middle?to=SplitToolModel, accessed on 23 April 2024) was used.
In vivo splicing analysis of CDH23 transcripts in the patient’s mother
Following prior confirmation of CDH23 expression in blood, samples from the patient’s mother and a control were utilized to analyze the splicing pattern of exons 22, 23, and 24 via RT-PCR. Their blood was collected in PAXgene Blood RNA tubes, and RNA was extracted using PAXgene Blood RNA Kit (PreAnalytiX, Hombrechtikon, Switzerland). Two rounds of PCR were performed using nested primers: the first PCR was performed using Complementary DNA (a total of two sets of DNA) as a template, with CDH23-1F (5-GAAATCACCACCACGTCTCT-3) and CDH23-1R (5-TGCTGCTGTTGATGAGGAAG-3) as primers for 30 cycles. The second PCR was performed using products from the first round of PCR as a template, with CDH23-2F (5-GTGGGCCACAACCAGAAAAC-3) and CDH23-2R (5-TTCAAGCACCTCGGCCACAA-3) as primers for 30 cycles.
Protein structure prediction
The protein sequence with 3354 amino acid residues of CDH23 was downloaded from uniprot web (https://www.uniprot.org/). The wild-type, mutant-type1 (CDH23:c.6068C > A) and mutant-type2 (CDH23:c.2398-6G > A) 3D structure of the CDH23 protein were predicted using AlphaFold web server (https://golgi.sandbox.google.com/about) (Abramson et al. 2024). The best model was selected based on pLDDTs prediction scores. The higher the score, the more confident is the structure. Prediction models editing was performed was visualized using PyMOL program (https://pymol.org/).
Bioinformatics analysis
The protein–protein interaction (PPI) network associated with CDH23 was constructed using STRING (https://string-db.org/). The amino acid sequence of the CDH23 protein, comprising 3354 residues, was retrieved from the UniProt database (https://www.uniprot.org/). This sequence was used as the input query for STRING, where the parameters were set to ensure high-confidence interactions were captured. The confidence score threshold was adjusted to include only interactions supported by strong evidence such as experimental data, co-expression, and co-occurrence analyses.
Results
Clinical description and audiological features
Given the association of various CDH23 mutations with usher syndrome, the patient underwent a thorough examination to rule out related phenotypes. For example, fundus examinations revealed no macular changes in both eyes. A comprehensive medical history was obtained using the previously mentioned questionnaire. Additionally, the patient did not exhibit other visual symptoms such as night blindness, visual field loss, or reduction in central vision. A CT of the temporal bone revealed normal pneumatization of both mastoids. The inner ear structures, including the cochleae, vestibules, semicircular canals, and middle ear structures, were all found to be normal. MR labyrinthography further confirmed that both the internal auditory canal and the labyrinth of the inner ear membrane were intact.
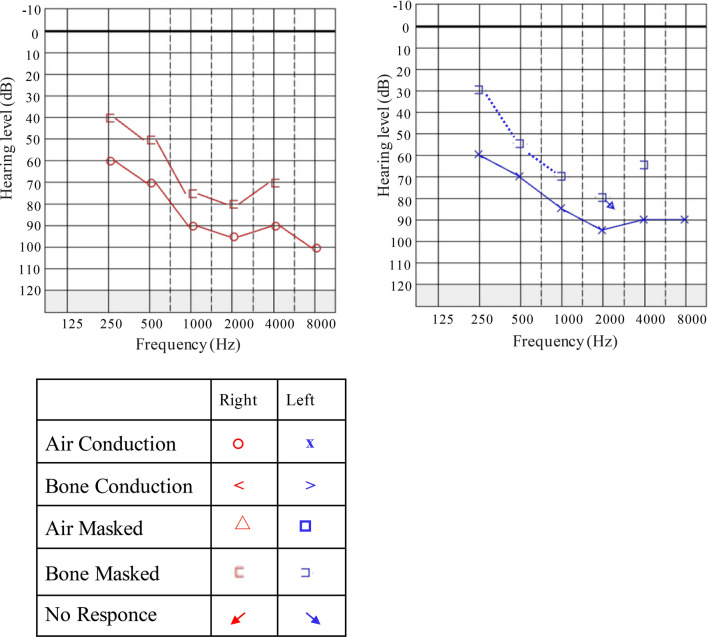
The patient exhibited a progressive nature of hearing loss, as confirmed by audiograms obtained at different ages. Audiological assessments, including tympanometry, DPOAE, AC-ABR and BC-ABR were conducted in her 7 and 7.5 years old, respectively (Table 2). ABR thresholds, which can predict pure-tone behavioral thresholds across a wide frequencies range (Gorga et al. 2006), revealed severe hearing loss in both ears at age 7. Six months later, the left ear hearing become worse up to profound hearing loss. What’s more, tympanometry at age 7.5 was showed flat curve at 1000 Hz in the left ear. By age 8, the average threshold of PTA air conduction was approximately 85 dB HL of two ears, indicating profound hearing loss (Fig. 1).
Table 2.
The outcomes of tympanometry, DPOAE, click-evoked AC- and BC-ABRs in 7 and 7.5 years old of the proband
| Age at test (year) | Ear | Tympanometry | DPOAE | AC-ABR 2000-4000 Hz (dBnHL) | AC-ABR 500 Hz (dBnHL) | AC-ABR 1000 HZ (dBnHL) | BC-ABR (dBnHL) |
|---|---|---|---|---|---|---|---|
| 7 | L | Positive peak | Refer | 70 | 65 | 75 | 45 |
| R | Positive peak | Refer | 70 | 65 | 75 | 45 | |
| 7.5 | L | Flat | Refer | 80 | 85 | 90 | 45 |
| R | Positive peak | Refer | 65 | 70 | 80 | 45 |
Fig. 1.
Pure Tone Audiometry (PTA) assessments reveal profound bilateral sensorineural hearing loss in the proband at 8 years of age. The audiometric data were graphically represented using Audiogram Creator (https://audiogramcreator.com) according to the original graphs. The legend symbol indicating various audiometric thresholds and conditions are shown above. The audiogram demonstrates the progression of profound hearing loss across both ears at age 8, with an average threshold of 85 dB HL for air conduction, indicating severe hearing impairment
WES and variant assessment
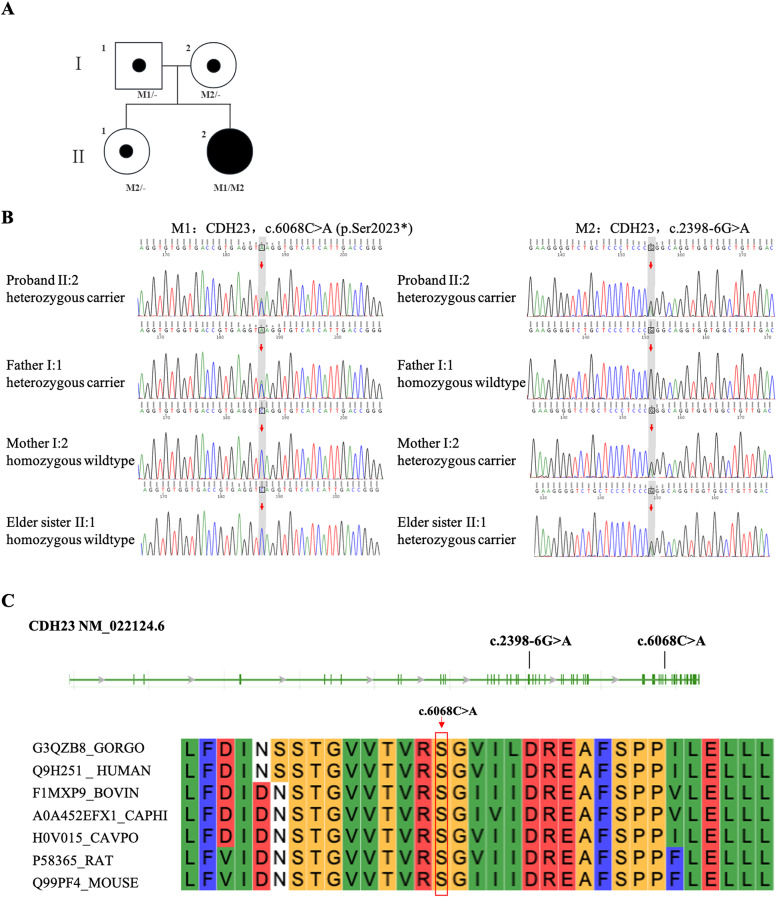
Trio-based WES was performed, which an average depth of coverage of 138 reads was obtained with 98% of targeted regions covered at ≥ 20 ×, and three variants, c.2398-6G > A and c.6068C > A in CDH23 (RefSeq NM_022124.6) and c.109G > A in GJB2 (RefSeq NM_004004.6), were considered as potential candidates. The pathogenic variants of CDH23 and GJB2 were related to the “hearing loss” and “regression of speech”. CDH23 mutations in compound heterozygotes were detected in the proband: c.2398-6G > A and c.6068C > A, which were inherited from the mother and father, respectively. The elder sister inherited c.2398-6G > A variant from the mother. Pedigree of the family with NSHL was shown in Fig. 2a. Both variants were confirmed by Sanger sequencing (Fig. 2b). The c.6068C > A (p. Ser2023*) is a nonsense variant in CDH23 that induces a premature stop codon in place of serine at position 2023. This serine residue is highly conserved across multiple species, including gorilla, human, bovine, goat, guinea pig, rat, and mouse, indicating its important role in maintaining the structural and functional integrity of the protein across different organisms (Fig. 2c). This variant lead to early termination of amino acid translation and may impact mRNA expression, which was not identified in population databases (1000 Genomes, Exome Aggregation Consortium, and Genome Aggregation Database). According to the American College of Medical Genetics and Genomics (ACMG) (Richards et al. 2015) guidelines for interpretation of sequence variants, we considered this variant to be likely pathogenic (PVS1 + PM2_Supporting). The c.2398-6G > A inherited her mother was present in GnomAD databases with extremely low minor allele frequency (MAF) (MAF = 6.574 × 10−5). In silico prediction indicated that the CDH23 c.2398-6G > A variant may affect slicing through activating a new acceptor site or causing exon skipping (Table 3).
Fig. 2.
Composite illustration detailing the genetic analysis of the study family. a The pedigree chart illustrates the inheritance pattern of NSHL within the family. Circles and squares denote females and males, respectively. The arrow indicates the proband. Fully shaded symbols represent individuals diagnosed with deafness, while partially shaded symbols denote carriers of CDH23 mutations. The genotypic data for the identified mutations in the CDH23 gene are indicated below each tested family member. CDH23: M1/- or M2/- indicate heterozygous carriers of the c.6068C > A, (p. Ser2023*) and c.2398-6G > A, respectively. CDH23: M1/M2 indicates compound heterozygous individuals. b Sanger sequencing results for the c.6068C > A and c.2398-6G > A mutations in all family members. Red arrows highlight the specific nucleotide positions of these mutations. The results demonstrated that the compound heterozygous mutation cosegregated with the HL phenotype, consistent with the recessive inheritance observed in the pedigree. c Diagram of the location of CDH23 mutations. The two identified mutations c.2398-6G > A and c.6068C > A are located in distinct regions of the gene. Key residues, particularly those at positions such as Ser2023, are shared across humans, gorillas, bovines, and rodents, suggesting their critical function in the protein’s structural integrity
Table 3.
The results of RDDCSC
| Predicted signal | Predicted splicing map | Interpretation |
|---|---|---|
| New Acceptor site |  |
Inserting 4 bp, premature termination, |
| Exon skipping |  |
Deleting 190 bp, premature termination |
Both splice patterns indicate the potential alteration of splicing in CDH23 c.2398-6G > A variant
Primitively, we considered this variant to be VUS (PM2 + PP3). The c.109G > A (p.Val37Ile) variants in GJB2 is classified as pathogenic for autosomal recessive nonsyndromic hearing loss, exhibiting variable expressivity and incomplete penetrance, according to the ClinGen Hearing Loss Expert Panel (Shen et al. 2019). The variants may lead to mild or moderate HI with reduced penetrance. Homozygous c.109G > A variants in GJB2 were detected in each member of this family including proband’s normal parents and sister.
Splicing analysis
In vivo RNA analysis further elucidated the impact of the heterozygous CDH23 c.2398-6G > A variant carried by the mother. RT-PCR analysis revealed the retention of four nucleotides (GCAG) between exon 22 and exon 23, which is not present in the normal control (Fig. 3a). This splice variant disrupts normal mRNA splicing, leading to an abnormal splice product with the insertion of these nucleotides. The splice variant: c.2398-6G > A affected the normal mRNA splicing, produce an abnormal splice product with the insertion of these nucleotides. Consequently, the cDNA representation of the variant is c.2397_2398insGCAG (p. Val800Alafs*6), leading to a frameshift mutation and premature termination codon (PTC) within exon 23 (Fig. 3b, c). This mutation likely produces a length of 804 truncated protein. According to the American College of Medical Genetics and Genomics (ACMG) (Richards et al. 2015) guidelines for interpretation of sequence variants, this variant is classified as likely pathogenic (PS3 + PM3 + PM2 + PP3).
Fig. 3.
In vivo functional analysis of splicing defects induced by CDH23 mutations. a Agarose gel electrophoresis of RT-PCR amplification products derived from the mother and a normal control, illustrating the differential splicing patterns. b Schematic overview of the RT-PCR primer design and the resultant splicing pattern. The red arrow indicates the mutation site that potentially disrupts normal splicing. c Sanger sequencing of RT-PCR products corresponding to shear bands a and b, and the red dashed box shows the retention of 4 nucleotides: GCAG between exon 22 and exon 23
Effect of mutations on protein structure
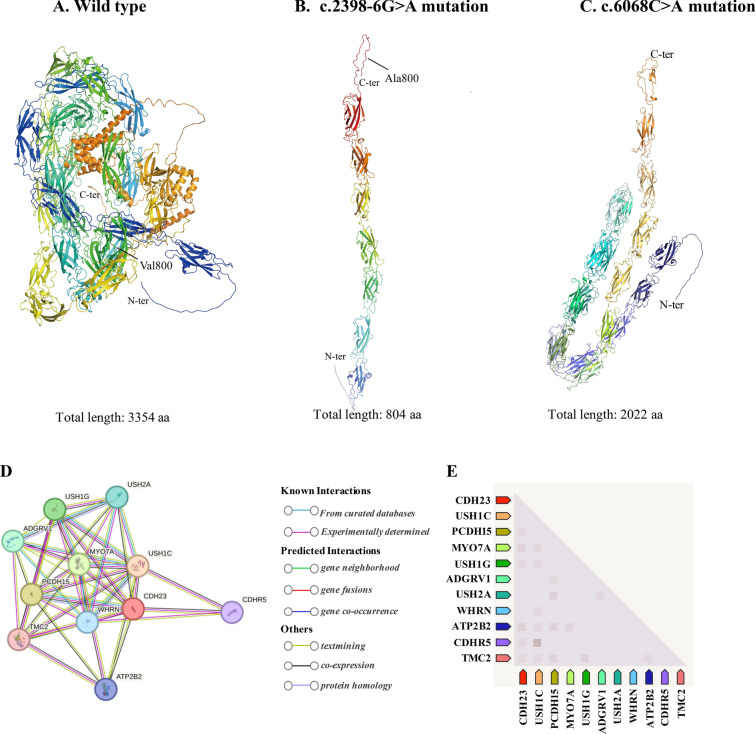
In order to analyze the effect of the two novel variants respectively, NM_022124.6: c.2398-6G > A, (p. Val800Alafs*6) and c.6068C > A, (p. Ser2023Ter) on protein structures CDH23, we used AlphaFold web server (https://golgi.sandbox.google.com/about) to predict changes in CDH23 protein structures when there were mutations in the sequence. The NM_022124.6: c.2398-6G > A variant in the CDH23 gene could induce a frameshift mutation, resulting in a truncated CDH23 protein comprising 804 of the 3354 amino acids of the mature protein. This frameshift mutation is likely to significantly alter the three-dimensional protein structure of CDH23 (Fig. 4b). The NM_022124.6: c.6068C > A, (p. Ser2023Ter) variant in CDH23 gene will change the three-dimensional structure to a truncated CDH23 protein that consist 2022 of the 3354 amino acids of the mature protein (Fig. 4c). This structural change would alter the conformation of the CDH23 protein and affect the protein stability and binding facility. The PPI network analysis of interacting genes with CDH23 was produced by STRING. As shown in Fig. 4d, e and Table 4, the PPI network consisted of 10 genes with interesting functional roles. Meanwhile, CDH23 expressed widely around the different tissue and localized mainly on postsynaptic region (Fig. 5a, b). Pathway analysis suggested that CDH23 and its interacting proteins are closely related to synaptic plasticity (Fig. 5c).
Fig. 4.
Structural modeling of CDH23 protein to assess the impact of mutations. a Predicted three-dimensional structure of the wild-type CDH23 protein, serving as the reference model. b Structural consequences of the c.2398-6G > A mutation on the CDH23 protein, indicating potential alterations in protein folding or function. c Structural impact of the c.6068C > A mutation on the CDH23 protein, illustrating how this mutation may compromise the protein’s integrity or interaction with other molecular components
Table 4.
Names and functions of predicted CDH23-interacting proteins
| Interacting proteins | Function of proteins | Co-expr.score |
|---|---|---|
| USH1C | Harmonin; USH1 protein network component harmonin | 0.999 |
| PCDH15 | Protocadherin-15; Calcium-dependent cell-adhesion protein. Essential for maintenance of normal retinal and cochlear function | 0.999 |
| MYO7A | Unconventional myosin-VIIa; Myosins are actin-based motor molecules with ATPase activity. Unconventional myosins serve in intracellular movements | 0.998 |
| USH1G | Usher syndrome type-1G protein; Anchoring/scaffolding protein that is a part of the functional network formed by USH1C, USH1G, CDH23 and MYO7A that mediates mechanotransduction in cochlear hair cells, which are required for normal hearing | 0.992 |
| ADGRV1 | Adhesion G-protein coupled receptor V1; G-protein coupled receptor which has an essential role in the development of hearing and vision. Couples to G-alpha(i)-proteins, GNAI1/2/3, G-alpha(q)-proteins, GNAQ, as well as G-alpha(s)-proteins, GNAS, inhibiting adenylate cyclase (AC) activity and cAMP production | 0.970 |
| USH2A | Usherin; Involved in hearing and vision as member of the USH2 complex. In the inner ear, required for the maintenance of the hair bundle ankle formation, which connects growing stereocilia in developing cochlear hair cells | 0.947 |
| WHRN | Whirlin; Involved in hearing and vision as member of the USH2 complex. Necessary for elongation and maintenance of inner and outer hair cell stereocilia in the organ of Corti in the inner ear. Involved in the maintenance of the hair bundle ankle region, which connects stereocilia in cochlear hair cells of the inner ear | 0.946 |
| ATP2B2 | Plasma membrane calcium-transporting ATPase 2; This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with the transport of calcium out of the cell | 0.900 |
| CDHR5 | Cadherin-related family member 5; CDHR5 interacts with microvillus cytoplasmic proteins, forming the intermicrovillar adhesion complex (IMAC), which is crucial for the differentiation of microvilli and the epithelial brush border | 0.881 |
| TMC2 | Transmembrane channel-like protein 2; Probable ion channel required for the normal function of cochlear hair cells. Component of the hair cell's mechanotransduction machinery. Involved in mechanosensitive responses of the hair cells | 0.875 |
Co-expr.score: Co-expression score
Fig. 5.
Bioinformatics analysis of the CDH23 gene expression, localization and networks. a GTEx data demonstrate that CDH23 is expressed at an appreciable level in adult human tissue represented (https://gtexportal.org/home/gene/CDH23). bc Subcellular localization and biological pathway were obtained through String data (https://string-db.org/cgi/network)
Discussion
Hearing loss adversely impacts language development, acquisition, and the social and cognitive maturation of affected children (Yang et al. 2023). In this study, the girl presented with hearing loss and regression of speech. Comprehensive medical histories and physical examinations, with a particular emphasis on audiological assessments, were conducted to exclude syndromic forms of hearing loss. Trio-based WES analysis was performed on the family, leading to the identification of two novel variants in the CDH23 gene.
DFNB12 arises from mutations in the CDH23 gene, located on chromosome 10q22, either in a homozygous or compound heterozygous state (Chaib et al. 1996). The non-syndromic form of DFNB12 hearing loss is usually linked to missense mutations in CDH23, which are believed to act as hypomorphic alleles. These alleles possess enough residual activity to maintain retinal and vestibular functions but fall short in supporting proper auditory function in the cochlea. A significant amount of research shows that USH1D is caused by homozygous nonsense, frameshift, and splice site mutations (primarily in canonical splice sites (Aparisi et al. 2013)), as well as certain missense mutations, or a combination of these mutations in a compound heterozygous configuration. (Bork et al. 2001a, 2002; Bolz and Roux 2011; Valero et al. 2019). Research has thoroughly established that USH1D is linked to homozygous nonsense, frameshift, and splice site mutations, especially those found at canonical splice sites, along with missense mutations in the CDH23 gene. Furthermore, a combination of these USH1D alleles in individuals with compound heterozygosity may also contribute to the development of the condition.
According to the human SNP pathogenic data on the Malacards website (https://www.malacards.org/), at least 15% of pathogenic mutations in the human SNP database are located within introns, with the majority of these intronic mutations occurring near exon–intron boundaries, primarily affecting mRNA splicing. Most pathogenic variants that disrupt splicing alter the canonical splice sites, but other variants located at the flanking regions, known as noncanonical splice sites (NCSS), are increasingly recognized as causes of aberrant splicing events. Intronic NCSS variants at splice donor sites are predominantly situated at positions + 3 to + 6 downstream from exons, while intronic NCSS variants at splice acceptor sites are located at positions − 14 to − 3 upstream of exons (Sangermano et al. 2018). These are often classified as variants of unknown VUS (Shaikh et al. 2018). The Human Gene Mutation Database indicated that NCSS and nonsense mutations in CDH23 could cause both USH1D and DFNB12 deafness (Valero et al. 2019). In this study, we identified two novel variants include NCSS and nonsense mutation, which led to the accurate diagnosis of the proband as DFNB12. It has been reported that two family members with compound heterozygous mutations in CDH23 resulting in DFNB12 have passed a newborn hearing screening, suggesting that their hearing loss is not congenital or was very mild at birth (Schultz et al. 2011). Similarly, the girl in our study also passed her newborn hearing screening. Wagatsuma et al. (2007) reported five unrelated Japanese families with DFNB12, all exhibiting a consistent phenotype characterized by moderate to profound high-frequency progressive sensorineural hearing loss. The average hearing loss among these patients was 84.0 dB HL, which closely aligns with our study's findings, where the proband's average hearing loss in both ears was approximately 85 dB HL.
CDH23 plays a crucial role in the proper organization of the static cilia bundle, and mutations in the CDH23 gene disrupting stereocilia structure. These disruptions impair the conversion of sound waves into electrical signals in hair cells, leading to noise-induced hearing loss and age-related hearing loss (Noben-Trauth et al. 2003). Yang et al. (2023) demonstrated that loss of CDH23 resulted in defective purine metabolism, subsequently insufficiency of ATP, which is of great importance for the normal function of hair cells. Patients with CDH23 mutations displayed a wide range of hearing loss and RP phenotypes, varying in severity, age at onset, type, and the presence or absence of vestibular areflexia (Astuto et al. 2002). In our study, the girl presented with bilateral profound sensorineural hearing loss but did not exhibit any additional phenotypes.
It is noteworthy that homozygous c.109G > A variants in GJB2 were identified in each member of this family including the patient’s unaffected parents and sister. Mutations in the gap-junction protein gene GJB2 (OMIM: 121,011) were the most common cause for autosomal recessive hearing impairment (HI) (Kelley et al. 1998). The GJB2 c.109G > A/p.Val37Ile has been increasingly studied recently due to its high prevalence in East Asians (Li et al. 2012; Kim et al. 2013), with an estimated 5 millions East Asians being homozygous for p.V37I variant (Chai et al. 2015). Initially, this variant was classified as a benign polymorphism for its high prevalence in normal hearing individuals (Kelley et al. 1998). However, recent studies have demonstrated that homozygous p.V37I can result in mild to moderate HI, albeit with reduced penetrance (Pollak et al. 2007; Gallant et al. 2013). Chai et al. (Chai et al. 2015) reported that homozygous p.V37I exhibits a relatively low penetrance (17%), with the majority of individuals carrying this variant avoiding significant hearing impairment until early adulthood. A large population screening in Shanghai revealed that biallelic p.V37I variant in GJB2 led to slow but steadily progressive hearing loss, with increasing incidence with age. Most individuals with the biallelic p.V37I likely develop significant hearing loss in adulthood. Notably, none of the individuals with homozygous c.109G > A variants in the 7- and 15-year-old groups exhibited moderate or severe (≥ 50 dB HL) hearing loss (Chen et al. 2022b). In our study, the two novel pathogenic variants in the CDH23 gene, rather than the homozygous c.109G > A variants, are responsible for the profound hearing loss (85 dB HL) observed in the 8-year-old girl. Patients with CDH23 mutations have been predicted to achieve satisfactory auditory and speech outcomes following cochlear implantation (Yoshimura et al. 2020; Chen et al. 2022a). It is anticipated that a cochlear implant will significantly enhance the hearing of the girl identified in our research. Therefore, identifying the genetic etiology of hearing loss in affected individuals is advantageous, as it can enhance prognostic accuracy in clinical settings. In conclusion, our analyses revealed novel compound heterozygous mutations in CDH23 associated with autosomal recessive NSHL, thereby expanding the mutational landscape of CDH23-related hearing loss and increasing knowledge about the CDH23 splice site variants.
Acknowledgements
The authors are grateful to the patient and their family members for their participation in this study.
Authors’ contributions
BL, WX, RL collected sequencing data and clinic information; QZ, JD, and TX analysed the data; BL, and WX draft the paper; HH and SH wrote the final paper. All authors contributed to the article and approved the submitted version.
Funding
Open access funding provided by University of Gothenburg. The research was supported by Fujian Provincial Natural Science Foundation of China (2021J02054).
Data availability
The authors confirm that the data supporting the findings of this study are available from the corresponding author on request.
Declarations
Ethics approval and consent to participate
This research was approved by the Ethics Committee of the Ganzhou Maternal and Child Health Hospital, Ganzhou, China. Informed consents were obtained from the subjects for all clinical studies.
Consent for publication
Informed consent was obtained from all subjects for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Baoqiong Liao and Wuming Xie have Contributed equally to this work.
Contributor Information
Shuwen He, Email: shuwen.he@gu.se.
Hailong Huang, Email: huanghailong@fjmu.edu.cn.
References
- Abramson J, Adler J, Dunger J et al (2024) Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630:493–500. 10.1038/s41586-024-07487-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford RL, Arnos KS, Fox M et al (2014) American College of Medical genetics and genomics guideline for the clinical evaluation and etiologic diagnosis of hearing loss. Genet Med off J Am Coll Med Genet 16:347–355. 10.1038/gim.2014.2 [DOI] [PubMed] [Google Scholar]
- Aparisi MJ, García-García G, Aller E et al (2013) Study of USH1 splicing variants through minigenes and transcript analysis from nasal epithelial cells. PLoS ONE 8:e57506. 10.1371/journal.pone.0057506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astuto LM, Bork JM, Weston MD et al (2002) CDH23 mutation and phenotype heterogeneity: a profile of 107 diverse families with Usher syndrome and nonsyndromic deafness. Am J Hum Genet 71:262–275. 10.1086/341558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz H, von Brederlow B, Ramírez A et al (2001) Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nat Genet 27:108–112. 10.1038/83667 [DOI] [PubMed] [Google Scholar]
- Bolz HJ, Roux A-F (2011) Clinical utility gene card for: Usher syndrome. Eur J Hum Genet EJHG. 10.1038/ejhg.2011.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork JM, Morell RJ, Khan S et al (2002) Clinical presentation of DFNB12 and Usher syndrome type 1D. Adv Otorhinolaryngol 61:145–152. 10.1159/000066829 [DOI] [PubMed] [Google Scholar]
- Bork JM, Peters LM, Riazuddin S et al (2001a) Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am J Hum Genet 68:26–37. 10.1086/316954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork JM, Peters LM, Riazuddin S et al (2001b) Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am J Hum Genet. 10.1086/316954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha S, Kamenov K, Cieza A (2021) The world report on hearing, 2021. Bull World Health Organ 99:242-242A. 10.2471/BLT.21.285643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Chen D, Sun L et al (2015) The homozygous p. V37I variant of GJB2 is associated with diverse hearing phenotypes. Clin Genet 87:350–355. 10.1111/cge.12387 [DOI] [PubMed] [Google Scholar]
- Chaib H, Place C, Salem N et al (1996) Mapping of DFNB12, a gene for a non-syndromal autosomal recessive deafness, to chromosome 10q21-22. Hum Mol Genet 5:1061–1064. 10.1093/hmg/5.7.1061 [DOI] [PubMed] [Google Scholar]
- Chen K, Huang B, Sun J et al (2022a) Cochlear implantation outcomes in children with cdh23 mutations-associated hearing loss. Otolaryngol-Head Neck Surg off J Am Acad Otolaryngol-Head Neck Surg 167:560–565. 10.1177/01945998211057427 [DOI] [PubMed] [Google Scholar]
- Chen Y, Wang Z, Jiang Y et al (2022b) Biallelic p. V37I variant in GJB2 is associated with increasing incidence of hearing loss with age. Genet Med 24:915–923. 10.1016/j.gim.2021.12.007 [DOI] [PubMed] [Google Scholar]
- Du YT, Cui QJ, Huang LH (2016) Progress in the clinical application of gene detection technology of deafness. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi J Clin Otorhinolaryngol Head Neck Surg 30:667–670. 10.13201/j.issn.1001-1781.2016.08.025 [DOI] [PubMed] [Google Scholar]
- Friedman TB, Schultz JM, Ahmed ZM et al (2011) Usher syndrome: hearing loss with vision loss. Adv Otorhinolaryngol 70:56–65. 10.1159/000322473 [DOI] [PubMed] [Google Scholar]
- Gallant E, Francey L, Tsai EA et al (2013) Homozygosity for the V37I GJB2 mutation in fifteen probands with mild to moderate sensorineural hearing impairment: further confirmation of pathogenicity and haplotype analysis in Asian populations. Am J Med Genet A 161A:2148–2157. 10.1002/ajmg.a.36042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga MP, Johnson TA, Kaminski JR et al (2006) Using a combination of click- and tone burst-evoked auditory brain stem response measurements to estimate pure-tone thresholds. Ear Hear 27:60–74. 10.1097/01.aud.0000194511.14740.9c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley PM, Harris DJ, Comer BC et al (1998) Novel mutations in the connexin 26 gene (GJB2) that cause autosomal recessive (DFNB1) hearing loss. Am J Hum Genet 62:792–799. 10.1086/301807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Park G, Han K-H et al (2013) Prevalence of p.V37I variant of GJB2 in mild or moderate hearing loss in a pediatric population and the interpretation of its pathogenicity. PLoS ONE 8:e61592. 10.1371/journal.pone.0061592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammens F, Verhaert N, Devriendt K et al (2013) Aetiology of congenital hearing loss: a cohort review of 569 subjects. Int J Pediatr Otorhinolaryngol 77:1385–1391. 10.1016/j.ijporl.2013.06.002 [DOI] [PubMed] [Google Scholar]
- Li L, Lu J, Tao Z et al (2012) The p.V37I exclusive genotype of GJB2: a genetic risk-indicator of postnatal permanent childhood hearing impairment. PLoS ONE 7:e36621. 10.1371/journal.pone.0036621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR (2003) Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet 35:21–23. 10.1038/ng1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olusanya BO (2012) Neonatal hearing screening and intervention in resource-limited settings: an overview. Arch Dis Child 97:654–659. 10.1136/archdischild-2012-301786 [DOI] [PubMed] [Google Scholar]
- Oshima A, Jaijo T, Aller E et al (2008) Mutation profile of the CDH23 gene in 56 probands with Usher syndrome type I. Hum Mutat 29:E37-46. 10.1002/humu.20761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak A, Skórka A, Mueller-Malesińska M et al (2007) M34T and V37I mutations in GJB2 associated hearing impairment: evidence for pathogenicity and reduced penetrance. Am J Med Genet A 143A:2534–2543. 10.1002/ajmg.a.31982 [DOI] [PubMed] [Google Scholar]
- Ramzan K, Al-Numair NS, Al-Ageel S et al (2020) Identification of novel CDH23 variants causing moderate to profound progressive nonsyndromic hearing loss. Genes 11:1474. 10.3390/genes11121474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med off J Am Coll Med Genet 17:405–424. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangermano R, Khan M, Cornelis SS et al (2018) ABCA4 midigenes reveal the full splice spectrum of all reported noncanonical splice site variants in Stargardt disease. Genome Res 28:100–110. 10.1101/gr.226621.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JM, Bhatti R, Madeo AC et al (2011) Allelic hierarchy of CDH23 mutations causing non-syndromic deafness DFNB12 or Usher syndrome USH1D in compound heterozygotes. J Med Genet 48:767–775. 10.1136/jmedgenet-2011-100262 [DOI] [PubMed] [Google Scholar]
- Shaikh SS, Nahorski MS, Rai H, Woods CG (2018) Before progressing from “exomes” to “genomes”… don’t forget splicing variants. Eur J Hum Genet EJHG 26:1559–1562. 10.1038/s41431-018-0214-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Oza AM, Del Castillo I et al (2019) Consensus interpretation of the p.Met34Thr and p.Val37Ile variants in GJB2 by the ClinGen Hearing Loss Expert Panel. Genet Med off J Am Coll Med Genet 21:2442–2452. 10.1038/s41436-019-0535-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJH, Bale JF, White KR (2005) Sensorineural hearing loss in children. Lancet Lond Engl 365:879–890. 10.1016/S0140-6736(05)71047-3 [DOI] [PubMed] [Google Scholar]
- Tb F, Jm S, Zm A et al (2011) Usher syndrome: hearing loss with vision loss. Adv Otorhinolaryngol. 10.1159/000322473 [Google Scholar]
- Valero R, de Castro-Miró M, Jiménez-Ochoa S et al (2019) Aberrant splicing events associated to CDH23 noncanonical splice site mutations in a proband with atypical Usher syndrome 1. Genes 10:732. 10.3390/genes10100732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagatsuma M, Kitoh R, Suzuki H et al (2007) Distribution and frequencies of CDH23 mutations in Japanese patients with non-syndromic hearing loss. Clin Genet 72:339–344. 10.1111/j.1399-0004.2007.00833.x [DOI] [PubMed] [Google Scholar]
- Yang S, Xie B-L, Dong X-P et al (2023) cdh23 affects congenital hearing loss through regulating purine metabolism. Front Mol Neurosci 16:1079529. 10.3389/fnmol.2023.1079529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura H, Moteki H, Nishio S-Y et al (2020) Genetic testing has the potential to impact hearing preservation following cochlear implantation. Acta Otolaryngol (Stockh) 140:438–444. 10.1080/00016489.2020.1730439 [DOI] [PubMed] [Google Scholar]
- Yuan Y, Li Q, Su Y et al (2020) Comprehensive genetic testing of Chinese SNHL patients and variants interpretation using ACMG guidelines and ethnically matched normal controls. Eur J Hum Genet EJHG 28:231–243. 10.1038/s41431-019-0510-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong LX (2013) Non-syndromic hearing loss and high-throughput strategies to decipher its genetic heterogeneity. J Otol. 10.1016/S1672-2930(13)50002-X [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available from the corresponding author on request.