Abstract
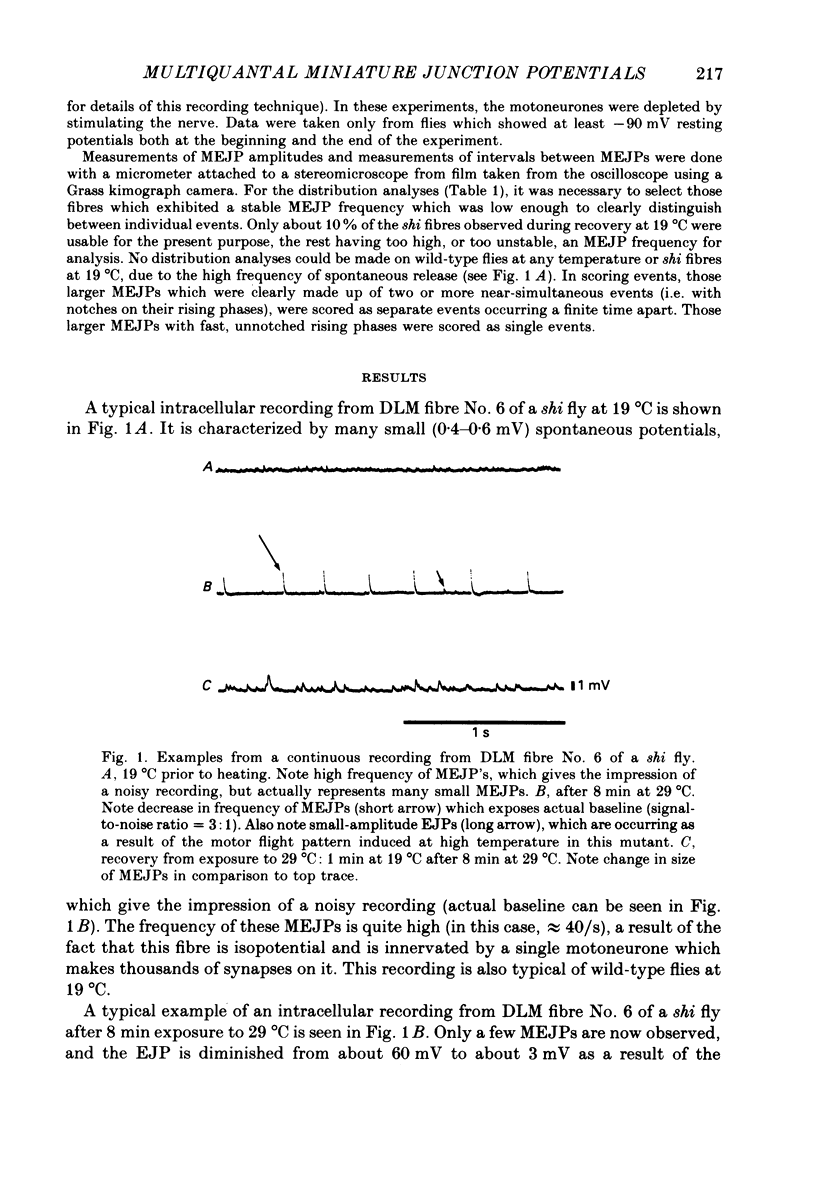
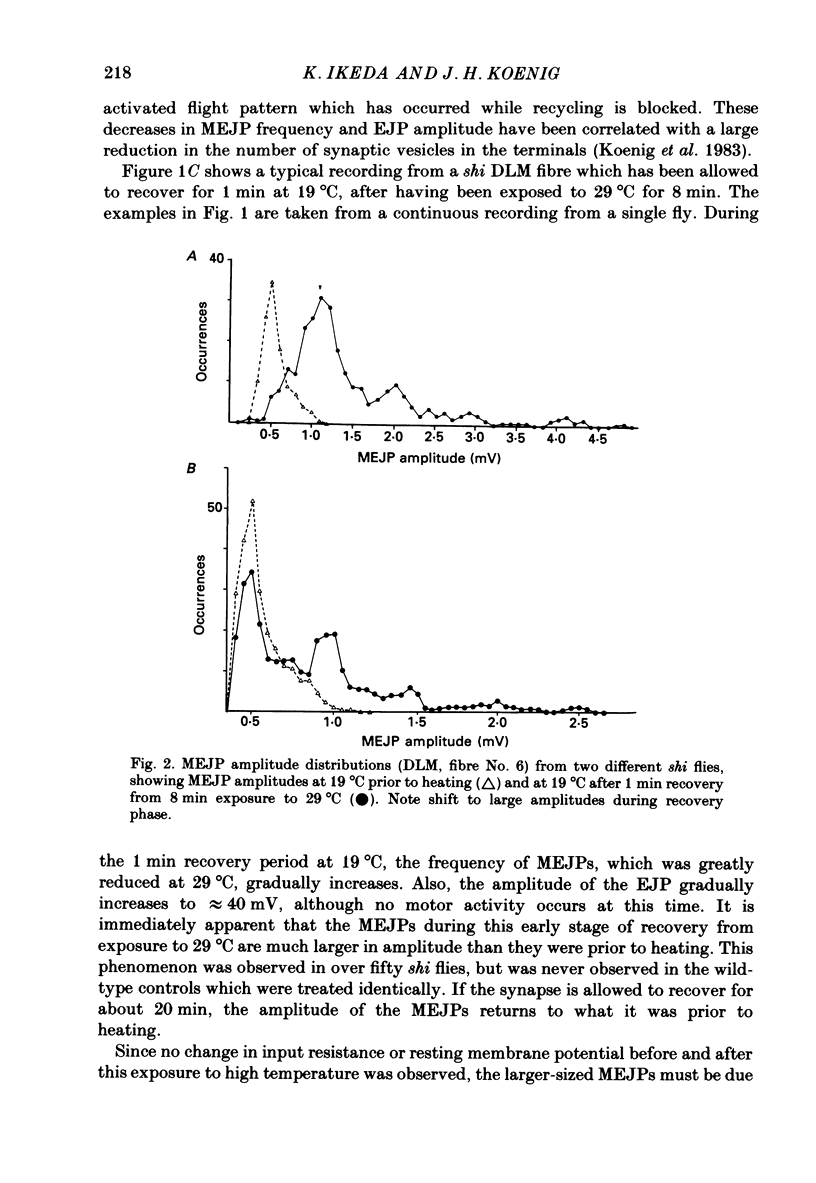
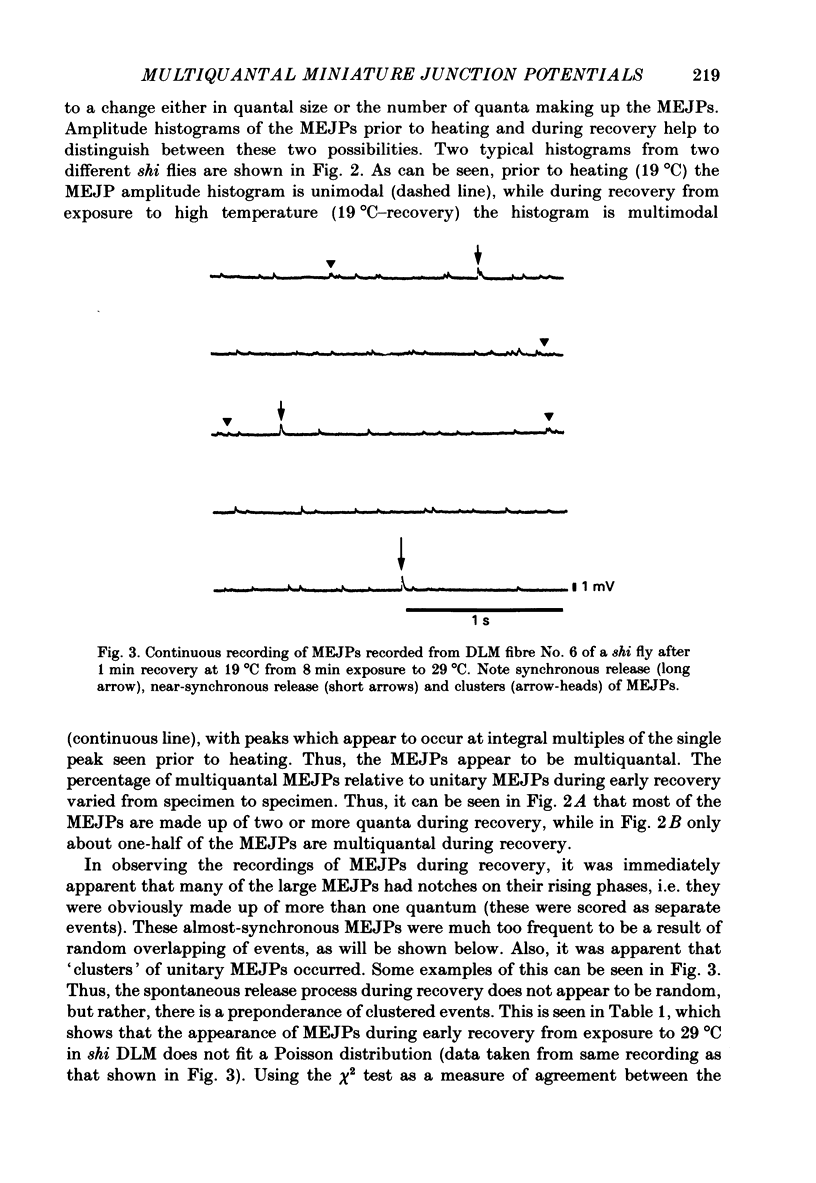
1. Intracellular recordings were made from muscle fibre No. 6 of the dorsal longitudinal flight muscle (DLM) of Drosophila melanogaster in both wild-type flies and the temperature-sensitive paralytic mutant, shibirets-1 (shi). 2. Continuous recordings of the miniature excitatory junction potentials (MEJPs) in this fibre were made as the temperature was changed from 19 to 29 degrees C, and back to 19 degrees C. In shi flies, synapses become depleted of vesicles at 29 degrees C due to a temperature-dependent blockage in the recycling process, while transmitter release proceeds normally. When the temperature is lowered to 19 degrees C, recycling is allowed to proceed and recovery of the full complement of synaptic vesicles gradually occurs in about 20 min. 3. It was observed that the MEJP amplitude distribution in shi flies was unimodal at 19 degrees C prior to heating (as was wild-type), but during recovery from 8 min exposure to 29 degrees C became multimodal, with peaks at roughly integral multiples of the original peak prior to heating. This effect was never seen in wild-type flies. 4. Also, during recovery, the MEJP did not occur randomly, but rather occurred in a clustered fashion. 5. It is concluded that during recovery from depletion in shi neuromuscular junctions, a condition exists which causes the synchronization of spontaneous release, causing multiquantal MEJPs or clustering of MEJPs, depending on the degree of synchronization. 6. The possible role of Ca2+ in this phenomenon is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine G. J., Levitan H. Neurotransmitter release and nerve terminal morphology at the frog neuromuscular junction affected by the dye Erythrosin B. J Physiol. 1983 Jan;334:47–63. doi: 10.1113/jphysiol.1983.sp014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in amphibian striated muscle during development. J Physiol. 1975 Oct;252(1):203–239. doi: 10.1113/jphysiol.1975.sp011141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S. Sub-miniature end-plate potentials at untreated frog neuromuscular junctions. J Physiol. 1976 Jun;258(1):145–155. doi: 10.1113/jphysiol.1976.sp011411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein J. C. Spontaneous multiquantal release at synapses in guinea-pig hypogastric ganglia: evidence that release can occur in bursts. J Physiol. 1978 Sep;282:375–398. doi: 10.1113/jphysiol.1978.sp012470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H. R., Martin A. R. Reduction in acetylcholine sensitivity of axotomized ciliary ganglion cells. J Physiol. 1976 Aug;260(1):159–175. doi: 10.1113/jphysiol.1976.sp011509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Kita H., Van Der Kloot W. The stochastic properties of spontaneous quantal release of transmitter at the frog neuromuscular junction. J Physiol. 1974 Jan;236(2):341–361. doi: 10.1113/jphysiol.1974.sp010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Fritz L. C., Atwood H. L., Jahromi S. S. Lobster neuromuscular junctions treated with black widow spider venom: correlation between ultrastructure and physiology. J Neurocytol. 1980 Oct;9(5):699–721. doi: 10.1007/BF01205034. [DOI] [PubMed] [Google Scholar]
- Heuser J. E. Proceedings: A possible origin of the 'giant' spontaneous potentials that occur after prolonged transmitter release at frog neuromuscular junctions. J Physiol. 1974 Jun;239(2):106P–108P. doi: 10.1113/jphysiol.1974.sp010593. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Koenig J. H., Tsuruhara T. Organization of identified axons innervating the dorsal longitudinal flight muscle of Drosophila melanogaster. J Neurocytol. 1980 Dec;9(6):799–823. doi: 10.1007/BF01205020. [DOI] [PubMed] [Google Scholar]
- Kelly S. S., Robbins N. Bimodal miniature and evoked end-plate potentials in adult mouse neuromuscular junctions. J Physiol. 1984 Jan;346:353–363. doi: 10.1113/jphysiol.1984.sp015027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J. H., Ikeda K. Flight pattern induced by temperature in a single-gene mutant of Drosophila melanogaster. J Neurobiol. 1980 Sep;11(5):509–517. doi: 10.1002/neu.480110510. [DOI] [PubMed] [Google Scholar]
- Koenig J. H., Ikeda K. Neural interactions controlling timing of flight muscle activity in Drosophila. J Exp Biol. 1980 Aug;87:121–136. doi: 10.1242/jeb.87.1.121. [DOI] [PubMed] [Google Scholar]
- Koenig J. H., Saito K., Ikeda K. Reversible control of synaptic transmission in a single gene mutant of Drosophila melanogaster. J Cell Biol. 1983 Jun;96(6):1517–1522. doi: 10.1083/jcb.96.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T., Ikeda K. Possible temperature-dependent blockage of synaptic vesicle recycling induced by a single gene mutation in Drosophila. J Neurobiol. 1983 May;14(3):207–225. doi: 10.1002/neu.480140305. [DOI] [PubMed] [Google Scholar]
- Kriebel M. E., Gross C. E. Multimodal distribution of frog miniature endplate potentials in adult denervated and tadpole leg muscle. J Gen Physiol. 1974 Jul;64(1):85–103. doi: 10.1085/jgp.64.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel M. E., Llados F., Matteson D. R. Spontaneous subminature end-plate potentials in mouse diaphragm muscle: evidence for synchronous release. J Physiol. 1976 Nov;262(3):553–581. doi: 10.1113/jphysiol.1976.sp011610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel M. E., Stolper D. R. Non-Poisson distribution in time of small- and large-mode miniature end-plate potentials. Am J Physiol. 1975 Nov;229(5):1321–1329. doi: 10.1152/ajplegacy.1975.229.5.1321. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. QUANTAL COMPONENTS OF THE SYNAPTIC POTENTIAL IN THE CILIARY GANGLION OF THE CHICK. J Physiol. 1964 Dec;175:1–16. doi: 10.1113/jphysiol.1964.sp007499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar P. C., Oen B. S., Polak R. L. Effect of chloride ions on giant miniature end-plate potentials at the frog neuromuscular junction. J Physiol. 1987 Feb;383:143–152. doi: 10.1113/jphysiol.1987.sp016401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pécot-Dechavassine M. Action of vinblastine on the spontaneous release of acetylcholine at the frog neuromuscular junction. J Physiol. 1976 Sep;261(1):31–48. doi: 10.1113/jphysiol.1976.sp011547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. The spontaneous release of transmitter from insect nerve terminals as predicted by the negative binomial theorem. J Physiol. 1974 Jan;236(1):129–142. doi: 10.1113/jphysiol.1974.sp010426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshenker S., Rahamimoff R. Neuromuscular synapse: stochastic properties of spontaneous release of transmitter. Science. 1970 Nov 6;170(3958):648–649. doi: 10.1126/science.170.3958.648. [DOI] [PubMed] [Google Scholar]
- Tauc L. Non vesicular release of neurotransmitter. Physiol Rev. 1982 Jul;62(3):857–893. doi: 10.1152/physrev.1982.62.3.857. [DOI] [PubMed] [Google Scholar]
- Usherwood P. N. Transmitter release from insect excitatory motor nerve terminals. J Physiol. 1972 Dec;227(2):527–551. doi: 10.1113/jphysiol.1972.sp010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig A., Stirner H. Quantum amplitude distributions point to functional unity of the synaptic 'active zone'. Nature. 1977 Oct 27;269(5631):820–822. doi: 10.1038/269820a0. [DOI] [PubMed] [Google Scholar]
- Wilson L., Bryan J., Ruby A., Mazia D. Precipitation of proteins by vinblastine and calcium ions. Proc Natl Acad Sci U S A. 1970 Jul;66(3):807–814. doi: 10.1073/pnas.66.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]


