Abstract
1. When inosine-fed human red cells are permeabilized to calcium by exposure to the ionophore A23187, progressively larger proportions of the cell population become irreversibly depleted of ATP as calcium influx is increased (Brown & Lew, 1983; García-Sancho & Lew, 1988b). When calcium influx is over 30 mmol/(l cells.h), all cells become ATP depleted and calcium equilibrated (E cells) (García-Sancho & Lew, 1988b). When calcium influx is lower, E cells co-exist with cells able to maintain normal ATP and low calcium contents in vigorous pump-leak balance (B cells). The experiments reported here investigate why calcium-induced ATP depletion of E cells is irreversible. 2. The inosine monophosphate (IMP) content of cells after 30 min of calcium permeabilization increased with the magnitude of the calcium load, roughly in inverse proportion to the fall in ATP. The calcium-induced increase in IMP was confined to the fraction of cells which became osmotically resistant after SCN- treatment (H cells), and which contained the E cells. 3. Cell nucleotides were measured after calcium permeabilization [( A23187]c = 100 mumol/l cells) in substrate-free media with different [Ca2+]o (0-0.5 mM). Calcium entry caused rapid ATP fall, AMP and IMP accumulation, and delayed ADP fall at all [Ca2+]o concentrations. Initial IMP formation increased with [Ca2+]o along a sigmoid saturation-like curve whereas AMP accumulation and ATP fall were maximal at [Ca2+]o = 20 microM and declined at the higher [Ca2+]o. The rate of IMP formation correlated positively with cell ATP and negatively with cell AMP at all [Ca2+]o values. 4. The AMP deaminase activity of red cell lysates was reversibly increased over tenfold by calcium. Half-maximal stimulation was observed at a Ca2+ concentration of about 50 microM. 5. These results suggest that the irreversibility of calcium-induced ATP depletion results from irreversible trapping of the adenine nucleotide as IMP, and help explain the mechanism of E cell formation.
Full text
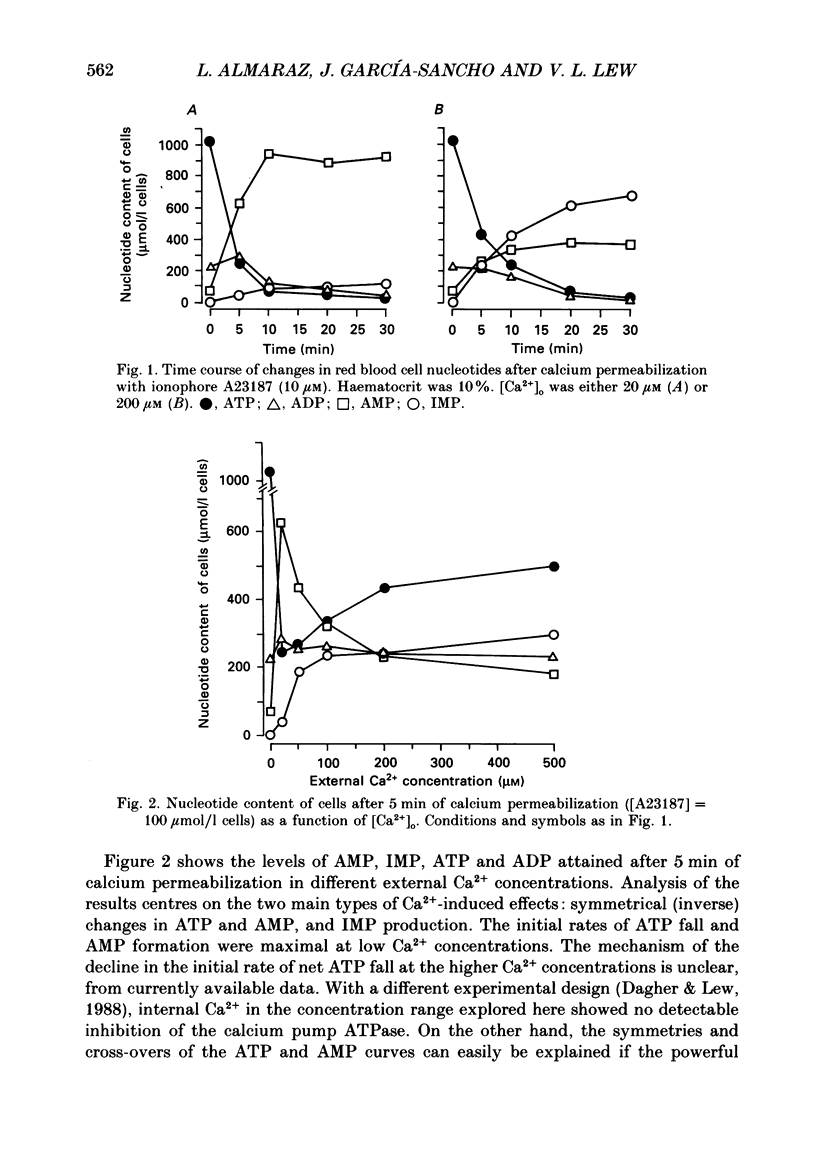
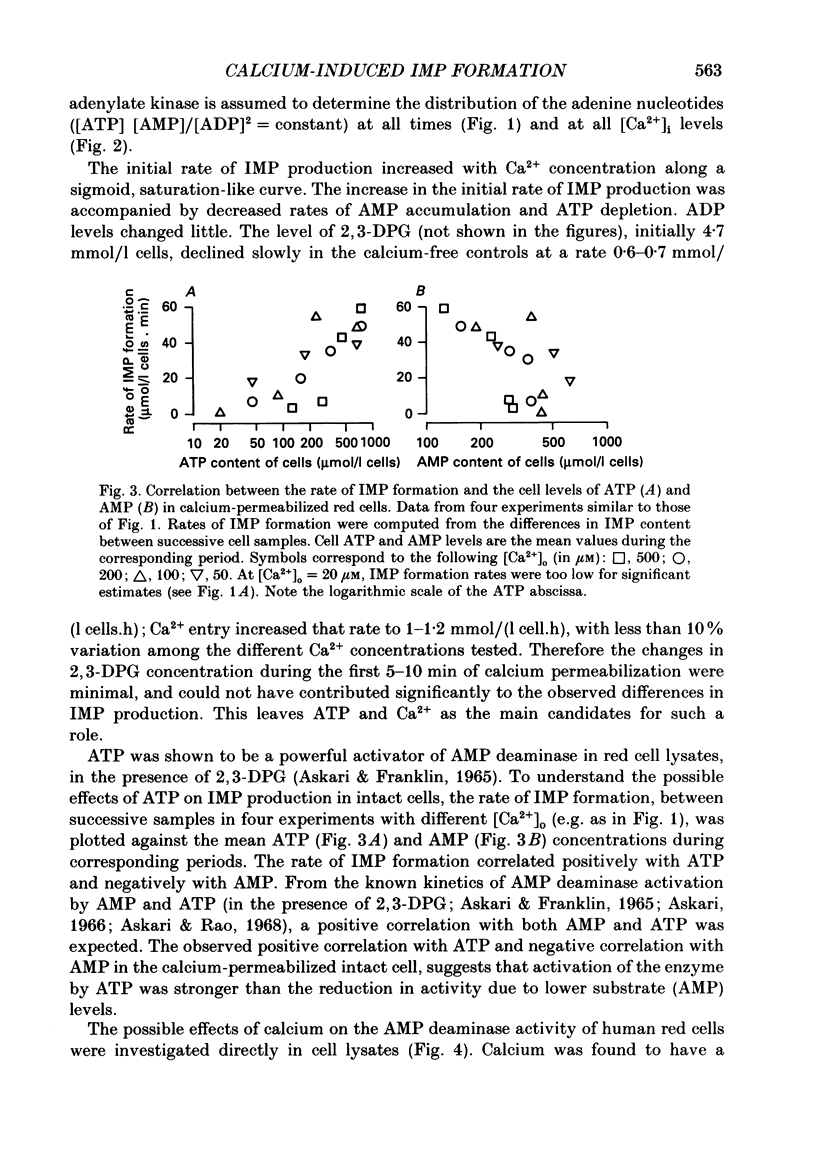
PDF



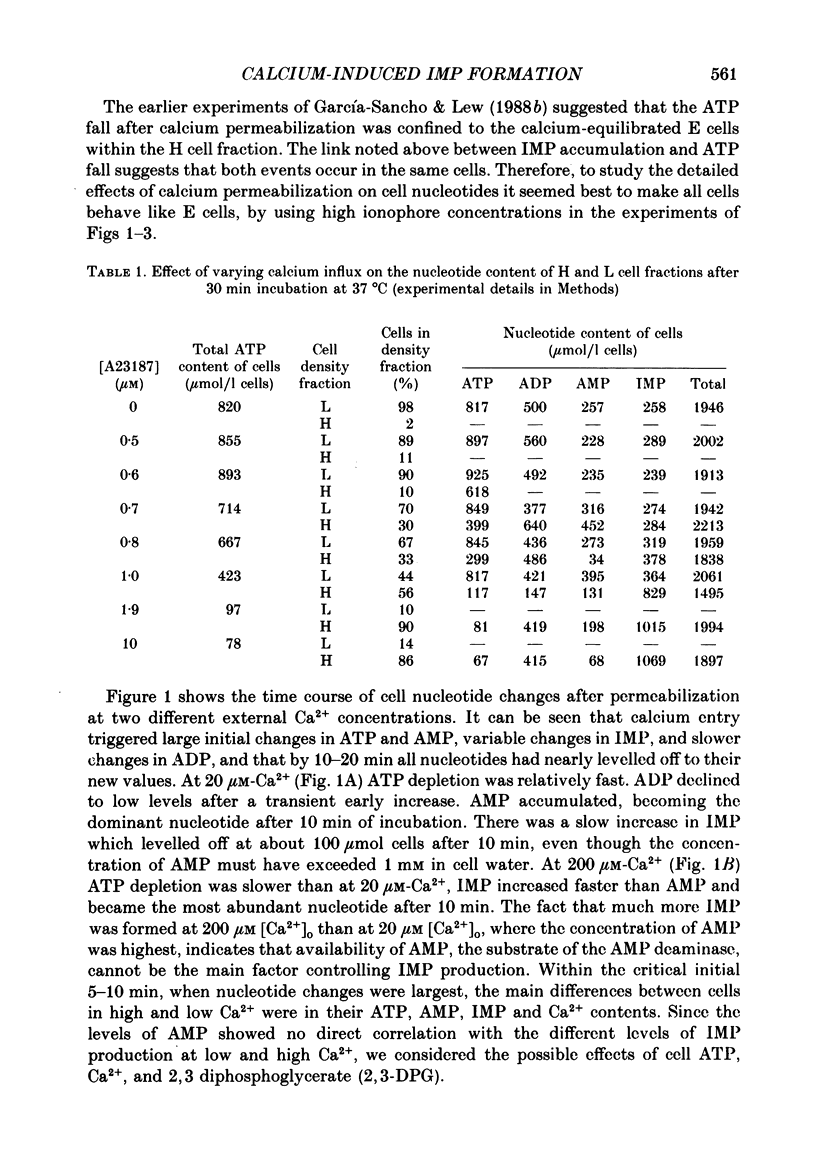
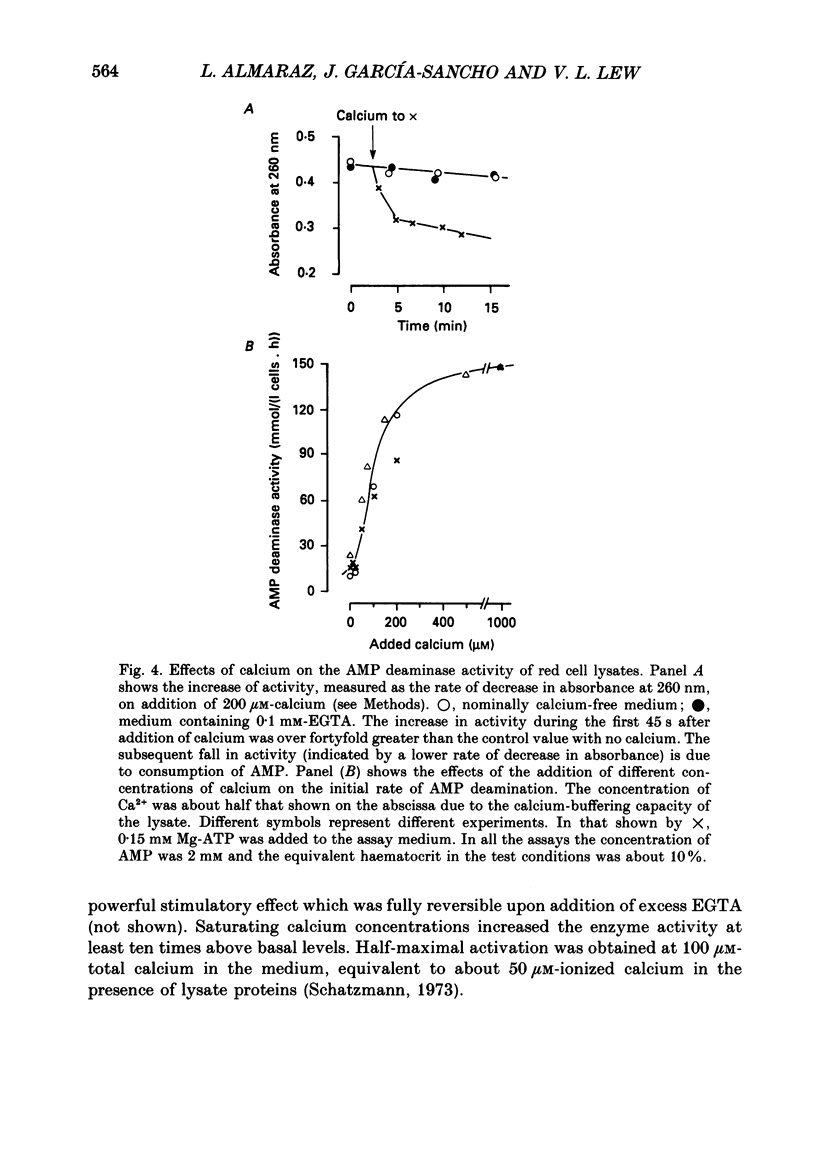



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askari A. Modifying effects of anions on the alkali-cation-activated AMP deaminase of human erythrocyte. Mol Pharmacol. 1966 Nov;2(6):518–525. [PubMed] [Google Scholar]
- Askari A., Rao S. N. Regulation of AMP deaminase by 2,3-diphosphoglyceric acid: a possible mechanism for the control of adenine nucleotide metabolism in human erythrocytes. Biochim Biophys Acta. 1968 Jan 8;151(1):198–203. doi: 10.1016/0005-2744(68)90174-5. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Lew V. L. The effect of intracellular calcium on the sodium pump of human red cells. J Physiol. 1983 Oct;343:455–493. doi: 10.1113/jphysiol.1983.sp014904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway E. J., Cooke R. The deaminases of adenosine and adenylic acid in blood and tissues. Biochem J. 1939 Apr;33(4):479–492. doi: 10.1042/bj0330479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher G., Lew V. L. Maximal calcium extrusion capacity and stoichiometry of the human red cell calcium pump. J Physiol. 1988 Dec;407:569–586. doi: 10.1113/jphysiol.1988.sp017432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira H. G., Lew V. L. Use of ionophore A23187 to measure cytoplasmic Ca buffering and activation of the Ca pump by internal Ca. Nature. 1976 Jan 1;259(5538):47–49. doi: 10.1038/259047a0. [DOI] [PubMed] [Google Scholar]
- García-Sancho J., Lew V. L. Detection and separation of human red cells with different calcium contents following uniform calcium permeabilization. J Physiol. 1988 Dec;407:505–522. doi: 10.1113/jphysiol.1988.sp017428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sancho J., Lew V. L. Heterogeneous calcium and adenosine triphosphate distribution in calcium-permeabilized human red cells. J Physiol. 1988 Dec;407:523–539. doi: 10.1113/jphysiol.1988.sp017429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The incorporation of inorganic phosphate into adenosine triphosphate by reversal of the sodium pump. J Physiol. 1967 Sep;192(1):237–256. doi: 10.1113/jphysiol.1967.sp008298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L., Lüthi U. Reversal of the potassium entry mechanism in red cells, with and without reversal of the entire pump cycle. J Physiol. 1970 Apr;207(2):371–391. doi: 10.1113/jphysiol.1970.sp009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L. Synthesis of adenosine triphosphate at the expense of downhill cation movements in intact human red cells. J Physiol. 1970 Apr;207(2):393–402. doi: 10.1113/jphysiol.1970.sp009068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE Y. P. 5'-Adenylic acid deaminase. III. Properties and kinetic studies. J Biol Chem. 1957 Aug;227(2):999–1007. [PubMed] [Google Scholar]
- Lew V. L., Glynn I. M., Ellory J. C. Net synthesis of ATP by reversal of the sodium pump. Nature. 1970 Feb 28;225(5235):865–866. doi: 10.1038/225865a0. [DOI] [PubMed] [Google Scholar]
- Lew V. L. On the ATP dependence of the Ca 2+ -induced increase in K + permeability observed in human red cells. Biochim Biophys Acta. 1971 Jun 1;233(3):827–830. doi: 10.1016/0005-2736(71)90185-4. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Wohlhueter R. M., Kraupp M. Adenine nucleotide metabolism and nucleoside transport in human erythrocytes under ATP depletion conditions. Biochim Biophys Acta. 1985 Jul 11;817(1):51–60. doi: 10.1016/0005-2736(85)90067-7. [DOI] [PubMed] [Google Scholar]
- Scharff O., Foder B. Delayed activation of calcium pump during transient increases in cellular Ca2+ concentration and K+ conductance in hyperpolarizing human red cells. Biochim Biophys Acta. 1986 Oct 23;861(3):471–479. doi: 10.1016/0005-2736(86)90456-6. [DOI] [PubMed] [Google Scholar]
- Scharff O., Foder B., Skibsted U. Hysteretic activation of the Ca2+ pump revealed by calcium transients in human red cells. Biochim Biophys Acta. 1983 May 5;730(2):295–305. doi: 10.1016/0005-2736(83)90346-2. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. Dependence on calcium concentration and stoichiometry of the calcium pump in human red cells. J Physiol. 1973 Dec;235(2):551–569. doi: 10.1113/jphysiol.1973.sp010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIL-MALHERBE H., GREEN R. H. Ammonia formation in brain. II. Brain adenylic deaminase. Biochem J. 1955 Oct;61(2):218–224. doi: 10.1042/bj0610218. [DOI] [PMC free article] [PubMed] [Google Scholar]


