Abstract
Aims
While few traditional scores are available for risk stratification of patients hospitalized for acute heart failure (AHF), the potential benefit of machine learning (ML) is not well established. We aimed to assess the feasibility and accuracy of a supervised ML model including environmental factors to predict in-hospital major adverse events (MAEs) in patients hospitalized for AHF.
Methods and results
In April 2021, a French national prospective multicentre study included all consecutive patients hospitalized in intensive cardiac care unit. Patients admitted for AHF were included in the analyses. A ML model involving automated feature selection by least absolute shrinkage and selection operator (LASSO) and model building with a random forest (RF) algorithm was developed. The primary composite outcome was in-hospital MAE defined by death, resuscitated cardiac arrest, or cardiogenic shock requiring assistance. Among 459 patients included (age 68 ± 14 years, 68% male), 47 experienced in-hospital MAE (10.2%). Seven variables were selected by LASSO for predicting MAE in the training data set (n = 322): mean arterial pressure, ischaemic aetiology, sub-aortic velocity time integral, E/e′, tricuspid annular plane systolic excursion, recreational drug use, and exhaled carbon monoxide level. The RF model showed the best performance compared with other evaluated models [area under the receiver operating curve (AUROC) = 0.82, 95% confidence interval (CI) (0.78–0.86); precision-recall area under the curve = 0.48, 95% CI (0.42–0.5), F1 score = 0.56). Our ML model exhibited a higher AUROC compared with an existing score for the prediction of MAE (AUROC for our ML model: 0.82 vs. ACUTE HF score: 0.57; P < 0.001).
Conclusion
Our ML model including in particular environmental variables exhibited a better performance than traditional statistical methods to predict in-hospital outcomes in patients admitted for AHF.
Study registration
ClinicalTrials.gov identifier: NCT05063097.
Keywords: Acute heart failure, Environmental factors, Carbon monoxide, Recreational drugs, Machine learning, Intensive cardiac care unit
Graphical Abstract
Graphical Abstract.
Prediction models using machine learning including environmental factors to assess the risk of in-hospital major adverse events in patients hospitalized for acute heart failure. ADDICT-ICCU, Addiction in intensive cardiac care unit; AHF, acute heart failure; CO, carbon monoxide; LASSO, least absolute shrinkage and selection operator; MAE, major adverse event; PR, precision-recall; TAPSE, tricuspid annular plane systolic excursion; TTE, transthoracic echocardiogram; LVOT VTI, left ventricular outflow tract velocity–time integral; XGBoost, extreme gradient boosting.
Introduction
Acute heart failure (AHF) continues to be the main cause for hospital admissions among patients over the age of 65 years and is responsible for a mortality exceeding 25%,1 including an 8% mortality rate within hospitals at the 30-day mark.2 It is therefore crucial to offer a high-performance prognostic stratification tool to identify these patients at risk of in-hospital poor outcomes. Beyond the clinical, biological, and echocardiographic parameters well established as prognostic factors,3,4 several recent studies have shown a role for environmental factors in the in-hospital prognosis of these AHF patients. Indeed, recent studies reported that recreational drug use could be a strong prognosticator of in-hospital outcomes due to multifactorial acute cardiotoxicity.5–7 Indeed, our working group has recently shown that recent consumption of recreational drugs in AHF patients was independently associated with a risk of in-hospital major adverse event (MAE) multiplied by more than seven compared with non-users.6 Another recent subanalysis showed that the level of exhaled CO, as a biomarker of air pollution in the patient's lungs, was independently associated with a higher risk of in-hospital mortality in AHF patients.8 Furthermore, a large meta-analysis emphasized the impact of air pollution, including carbon monoxide (CO) due to active and passive smoking, as an aggravating factor of AHF.9 However, until now, no tool is available to take into account all of these variables simultaneously.
Machine learning (ML) methods can recognize patterns in large data sets with a multiplicity of variables and can be used for improving risk stratification. Recently, ML techniques, using supervised or unsupervised approaches, have emerged as highly effective methods for prognostic prediction and decision-making in several cardiovascular diseases.10,11 Compared with unsupervised ML, a supervised ML method overcomes the main limitations of traditional methods with the problems of collinearity, inadequate model complexity, and violation of rigid assumptions. In addition, the strength of supervised ML is its ability to approximate non-linear relationships between hundreds of variables. Although of potential interest, supervised ML techniques using all this spectrum of variables have not been evaluated for risk stratification in AHF patients.
Therefore, the aim of this study was to investigate the feasibility and accuracy of a supervised ML model using clinical, biological, environmental, and echocardiographic data to predict in-hospital MAE in a consecutive cohort of patients hospitalized for AHF.
Methods
Study population
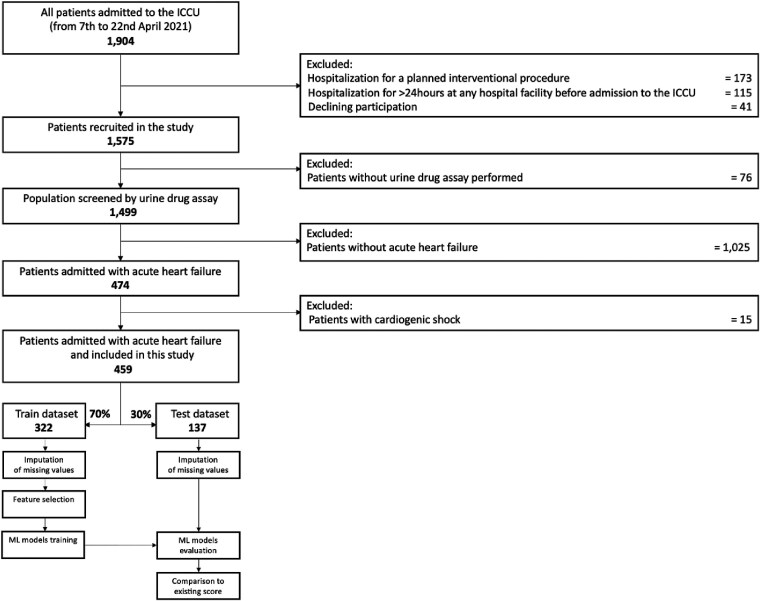
All details of the Addiction in Intensive Cardiac Care Units (ADDICT-ICCU) study design have been published previously.12 Briefly, this is a multicentre, prospective, observational study of all consecutive patients aged ≥18 years admitted to intensive cardiac care units (ICCUs) over 2 weeks in April 2021 at 39 centres across France (including all diagnoses). The main exclusion criterion was hospitalization for either a planned interventional procedure or more than 24 h at any hospital facility before admission to the ICCU. This was to prevent the risk of obtaining a negative urine drug assay in patients with recreational drug consumption more than 24 h prior to admission. The methodology of the baseline characteristics collection is detailed in Supplementary material online, Supplement S1. In this study, we decided to focus the analysis on patients admitted for AHF defined by symptoms and/or signs of heart failure with evidence of diastolic or systolic dysfunction by echocardiography and elevated levels of natriuretic peptide [B-type natriuretic peptide (BNP) > 35 pg/mL and/or N-terminal prohormone of BNP (NT-proBNP) > 125 pg/mL]3 (Figure 1). The main admission diagnosis was adjudicated by two independent experts at the end of the hospitalization following the current guidelines (see Supplementary material online, Supplement S2). The investigation conforms with the principles outlined in the Declaration of Helsinki. All patients had a written informed consent obtained at enrolment and were informed of the urine drug assay and exhaled CO measurement. This study has been approved by the Ethics Committee (Committee for the Protection of Human Subjects, Ile de France-7, France) and registered on the ClinicalTrials.gov website under the number NCT05063097.
Figure 1.
Flowchart of the study population. ICCU, intensive cardiac care unit; ML, machine learning.
Primary outcome
The primary outcome was in-hospital MAE defined as a composite of all-cause mortality, cardiogenic shock (requiring medical or mechanical haemodynamic support) and resuscitated cardiac arrest (severe ventricular arrhythmia requiring defibrillation or anti-arrhythmic agents). An independent committee of experts was in charge of reviewing anonymized medical documents according to standardized definitions.13
Baseline characteristics collection
The database records of the AHF population comprise baseline data that include clinical, demographic, reason for hospitalization, list of medications especially cardiovascular medications at admission, and history of cardiovascular disease. Blood pressure was measured non-invasively. Biological data were collected systematically upon admission, including haemoglobin, serum potassium, creatinine, the peak of troponin (hsTNI), the NT-proBNP or BNP. Standardized transthoracic echocardiography was performed systematically within the first 24 h of admission for all patients by a cardiologist and reviewed by a senior.
Regarding environmental factors, the presence of recreational drugs was determined through urine analysis using a dedicated drug assay (NarcoCheck®, Kappa City Biotech SAS, Montluçon, France) within 2 h of admission to the ICCU with excellent performance (the sensitivity and specificity of the urine drug assay were 91.7% and 97.7%, respectively).6 The following recreational drugs will be screened for all patients: cannabinoids (tetrahydrocannabinol), including cannabis and hashish; cocaine and metabolites, including cocaine and crack; amphetamines; 3,4-methylenedioxy-methylamphetamine (or ecstasy); and heroin and other opioids. Finally, a standardized exhaled CO measurement was systematically performed with a CO-Check Pro device (Micro Direct Diagnostics Ltd, UK) immediately on arrival in ICCU.
Machine learning
Fourteen clinical, four biological, three environmental, and seven echocardiographic parameters were available (detailed list provided in Supplementary material online, Supplement S3). Machine learning involved feature selection by least absolute shrinkage and selection operator (LASSO) algorithm and model building with random forest algorithm following guidelines for ML.14
Feature selection
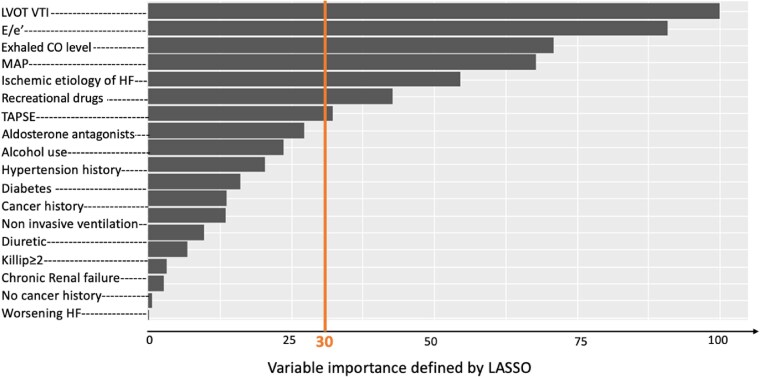
After the imputation of missing values by the K-nearest neighbours with a value of K = 5, feature selection was performed using LASSO (Figure 2), which is a penalized logistic regression that reduces the number of variables (glmnet R package).15 The regularization parameter λ controls the strength of the shrinkage to get the best compromise between prediction performance and interpretability. The best λ was found using 10-fold cross-validation and the ‘1 standard error rule’, for which the area under the receiver operating curve (AUROC) of the most parsimonious model is no more than 1 standard error above the AUROC of the best model.16
Figure 2.
Feature importance selected by least absolute shrinkage and selection operator. Least absolute shrinkage and selection operator was used to evaluate the worth of each variable by measuring the log-rank–based variable importance with respect to the outcome and then to rank the attributes by their individual evaluations (top to bottom). Only attributes resulting in log-rank based variable importance > 30 (above the vertical line) were subsequently used for the model building. CO, carbon monoxide; HF, heart failure; LASSO, least absolute shrinkage and selection operator; LVOT VTI, left ventricular outflow tract velocity–time integral; MAP, mean arterial; TAPSE, tricuspid annular plane systolic excursion.
Model building
To build the prediction model based on the training cohort, we compared four modelling strategies with logistic regression, random forest, extreme gradient boosting (XGBoost) model, and LASSO. To assess the performance of these algorithms, we used several metrics: AUROC, precision-recall area under the curve (PRAUC), F1 score, sensitivity, specificity, positive and negative prediction value, accuracy, balanced accuracy, Cohen’s kappa, Brier score, and McNemar test P-value. Due to the low occurrence of events and the imbalanced nature of the data, the F1 score and the precision-recall curve were preferred to determine the best model to minimize the overall misclassification cost and mitigate the problem of imbalanced data. For the prediction of in-hospital MAE, the best model was the ML model using random forest (Figure 3). Random forest corresponds to a method based on decision trees, where each tree is trained on a random sample from the data set. All the results presented are shown using only the testing cohort for validation.
Figure 3.
Performance of machine learning models for prediction of in-hospital major adverse event. Receiver operating characteristic (area under the receiver operating curve, A) and precision-recall curves (precision-recall area under the curve, B) for prediction of in-hospital MAE were used to compare least absolute shrinkage and selection operator, random forest, logistic regression, and extreme gradient boosting, developed using the set of variables selected by least absolute shrinkage and selection operator. AUC, area under the curve; LASSO, least absolute shrinkage and selection operator; ROC, receiver operating curve; XGBoost, extreme gradient boosting.
For model development, patients hospitalized for AHF in the ADDICT-ICCU study were randomly partitioned into training (70%) and validation (30%) cohorts (see Supplementary material online, Supplement S4). We used a 10-fold cross-validation process to tune the model hyperparameters using grid search.
Statistical analysis
Continuous data will be reported as mean ± standard deviation for normally distributed data or as medians and interquartile range for non-normally distributed data. Categorical data will be reported as counts and percentages. Between-group comparisons will be performed using the Student’s t-test or Mann–Whitney test for continuous variables and the χ2 or Fisher’s exact test for categorical variables, as appropriate.
Model discrimination was measured using the AUROC and its 95% confidence intervals (CIs). In addition, the positive predictive value (or precision) and the sensitivity (recall) across all possible risk thresholds for predicting in-hospital MAE were plotted using precision-recall curves and their area under the curve. The PRAUC, unlike the AUROC, is not affected by the number of true-negative results. In data sets with small event rates and therefore a large expected true-negative rate, such as the current study, the PRAUC is better for comparing different models. For both the AUROC and PRAUC, values closer to 1 correspond to more accurate models. DeLong's test was used to compare the AUROC of each model. We also calculated the F1 score, sensitivity, specificity, positive predictive value, and negative predictive value. In addition, we calculated a Brier score for each model as a measure of model accuracy.
Model calibration was measured using (i) the Hosmer–Lemeshow goodness-of-fit test; (ii) the reliability component of the Brier score; and (iii) graphically with plots showing the observed and predicted proportion of events, grouped by level of risk.
A two-tailed P-value < 0.05 will be considered statistically significant. All data will be analysed using R software, version 4.0.3 (R Project for Statistical Computing, R Foundation, Vienna, Austria).
Results
Study population
Detailed flowchart of the study is depicted in Figure 1. Among the whole population of the study, 459 patients were admitted for AHF (68% male, mean age 68 ± 14 years), 46% had ischaemic cardiomyopathy and 53% with LVEF value < 50%. All characteristics of the population are described in Table 1. Concerning the main admission diagnosis associated with AHF, 135 (29%) patients had acute coronary syndromes, 39 (9%) had severe conduction/arrhythmia abnormalities, 29 (6%) had pulmonary embolism, 20 (4%) had acute myocarditis, 9 (2%) had Takotsubo syndrome, 6 (1%) had spontaneous coronary dissection, 24 (5%) had other cardiovascular diagnoses, and 197 (44%) had isolated AHF.
Table 1.
Baseline characteristics according to the occurrence of in-hospital major adverse events (N = 459)
| All patients (n = 459) | Patients without MAE (n = 412) | Patients with MAE (n = 47) | P-value | |
|---|---|---|---|---|
| Demographic data | ||||
| Age, years | 68 ± 14 | 68 ± 14 | 70 ± 15 | 0.37 |
| Males, n (%) | 311 (67.8%) | 276 (67.0%) | 35 (74.5%) | 0.30 |
| Body mass index, kg/m² | 28 ± 6 | 28 ± 7 | 27 ± 5 | 0.32 |
| Cardiovascular risk factors, n (%) | ||||
| Diabetes mellitus | 131 (28.5%) | 112 (27.2%) | 19 (40.4%) | 0.06 |
| Hypertension | 287 (62.5%) | 255 (61.9%) | 32 (68.1%) | 0.41 |
| Dyslipidaemia | 204 (44.4%) | 183 (44.4%) | 21 (44.7%) | 0.97 |
| Current or previous smoking | 285 (63.1%) | 255 (63.0%) | 30 (63.8%) | 0.91 |
| Chronic obstructive pulmonary disease | 38 (8.3%) | 32 (7.8%) | 6 (12.8%) | 0.26 |
| History of CV disease, n (%) | ||||
| Known CAD | 107 (23.3%) | 93 (22.6%) | 14 (29.8%) | 0.27 |
| Chronic renal failure | 96 (20.9%) | 85 (20.6%) | 11 (23.4%) | 0.66 |
| History of HF hospitalization | 65 (14.2%) | 58 (14.1%) | 7 (14.9%) | 0.88 |
| Clinical parameters at admission | ||||
| Mean arterial pressure, mmHg | 98 ± 21 | 100 ± 20 | 84 ± 19 | <0.001 |
| Heart rate, b.p.m. | 91 ± 27 | 90 ± 27 | 96 ± 28 | 0.17 |
| Oxygen saturation, % | 96.3 ± 3.6 | 96.4 ± 3.6 | 95.9 ± 3.5 | 0.22 |
| Killip class | ||||
| I | 216 (47.1%) | 194 (47.1%) | 22 (46.8%) | 0.97 |
| II | 169 (36.8%) | 151 (36.7%) | 18 (38.3%) | 0.82 |
| III | 74 (16.1%) | 67 (16.3%) | 7 (14.9%) | 0.81 |
| Main admission diagnosis | 412 | 47 | ||
| Isolated AHF | 197 (43%) | 185 (45%) | 12 (26%) | <0.001 |
| Acute coronary syndrome | 135 (29%) | 117 (29%) | 18 (38%) | <0.001 |
| Conduction abnormalities/arrhythmia | 39 (9%) | 37 (9%) | 2 (4%) | 0.12 |
| Pulmonary embolism | 29 (6%) | 25 (6%) | 4 (9%) | 0.42 |
| Acute myocarditis | 20 (4%) | 16 (4%) | 4 (7%) | 0.09 |
| Takotsubo syndrome | 9 (2%) | 8 (2%) | 1 (2%) | 0.87 |
| Coronary dissection | 6 (1%) | 4 (1%) | 2 (4%) | 0.67 |
| Other CV diagnoses | 24 (5%) | 17 (5%) | 4 (8%) | 0.12 |
| Laboratory results | ||||
| Haemoglobin, g/dL | 12.9 ± 2.2 | 12.9 ± 2.2 | 12.4 ± 2.4 | 0.18 |
| Creatinemia, µmol/L | 118 ± 91 | 116 ± 91 | 128 ± 83 | 0.15 |
| High-sensitivity cardiac troponin peak, Ul/L | 354 ± 1906 | 335 ± 1979 | 516 ± 1079 | <0.001 |
| NT-proBNP, pg/mL | 13 960 ± 23 843 | 13 815 ± 24 401 | 15 233 ± 18 396 | 0.145 |
| Echocardiography data | ||||
| LA dilatation ≥ 32 mL/m², n (%) | 144 (31.4%) | 123 (29.9%) | 21 (44.7%) | 0.038 |
| Mitral E/A ratio | 1.32 ± 0.71 | 1.30 ± 0.72 | 1.49 ± 0.55 | <0.001 |
| Mitral E/e′ ratio | 10.7 ± 4.2 | 10.3 ± 3.8 | 13.9 ± 5.6 | <0.001 |
| LVEF, % | 45 ± 16 | 46 ± 16 | 37 ± 17 | <0.001 |
| LVEDV, mL/m² | 124 ± 57 | 122 ± 56 | 135 ± 57 | 0.089 |
| LVOT VTI, cm | 17.7 ± 5.9 | 18.1 ± 5.7 | 14.2 ± 5.8 | <0.001 |
| sPAP, mmHg | 31 ± 15 | 31 ± 15 | 34 ± 14 | 0.048 |
| RV dilation, n (%) | 63 (13.7%) | 58 (14.1%) | 5 (10.6%) | 0.52 |
| TAPSE, mm | 19.2 ± 4.8 | 19.4 ± 4.7 | 16.6 ± 4.8 | <0.001 |
| Heart failure data | ||||
| Worsening heart failure | 95 (20.7%) | 86 (20.9%) | 9 (19.1%) | 0.78 |
| Ischaemic aetiology of heart failure | 212 (46.2%) | 180 (43.7%) | 32 (68.1%) | 0.001 |
| Type of heart failure | 0.011 | |||
| HFrEF | 155 (33.8%) | 131 (31.8%) | 24 (51.1%) | |
| HFmEF | 87 (19.0%) | 77 (18.7%) | 10 (21.3%) | |
| HFpEF | 217 (47.3%) | 204 (49.5%) | 13 (27.7%) | |
| Previous treatment | ||||
| Aldosterone antagonists | 44 (9.6%) | 35 (8.5%) | 9 (19.1%) | 0.032 |
| ACEI/ARB, or Entresto | 219 (47.7%) | 195 (47.3%) | 24 (51.1%) | 0.63 |
| Diuretics | 136 (29.6%) | 117 (28.4%) | 19 (40.4%) | 0.09 |
| Environmental factors | ||||
| Alcohol consumption | 230 (50.1%) | 210 (51.0%) | 20 (42.6%) | 0.27 |
| CO, ppm | 4.5 ± 5.1 | 4.2 ± 4.4 | 7.8 ± 8.5 | 0.026 |
| Recreational drug use, n (%) | 42 (9.2%) | 30 (7.3%) | 12 (25.5%) | <0.001 |
Values are n (%), mean ± SD, or median (interquartile range).
ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; CAD, coronary artery disease; CO, carbone monoxide; CV, cardiovascular; HF, heart failure; HFrEF, heart failure reduced ejection fraction; HFmEF, heart failure mild ejection fraction; HFpEF, heart failure preserved ejection fraction; LA, left atrium; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end diastolic volume; LVOT VTI, left ventricular outflow tract velocity–time integral; MAE, major adverse event; NT-proBNP, N-terminal prohormone of B-type natriuretic peptide; RV, right ventricular; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion
During a median duration of hospitalization of 7 days (interquartile range 5–12 days) in the ICCU, 47 patients (10.2%) experienced in-hospital MAE, including 18 (3.9%) in-hospital deaths, 8 (1.7%) resuscitated cardiac arrests, and 21 (4.6%) cardiogenic shocks requiring medical and/or mechanical haemodynamic support. Patients who experienced in-hospital MAE had a lower mean arterial pressure, a higher cardiac troponin level, a lower LVEF value, and tricuspid annular plane systolic excursion (TAPSE) value compared with patients without in-hospital MAE (all P < 0.001).
Feature selection
Using the LASSO feature selection method on the training cohort with the best regularization parameter λ (see Supplementary material online, Supplement S5), seven of the available variables were selected for the ML model (two clinical, two environmental, and three echocardiographic) in descending order of importance: left ventricular outflow tract velocity–time integral (LVOT VTI), peak E/e′ ratio, exhaled CO level, mean arterial pressure, ischaemic AHF aetiology, recreational drug use, and TAPSE (Figure 2).
Model building
The ML model using random forest exhibited the best global performance for the prediction of in-hospital MAE in the testing cohort compared with LASSO, stepwise regression, and XGBoost using all the metrics available (Table 2).
Table 2.
Performance of some evaluated machine learning models for predicting in-hospital major adverse event
| Evaluation metrics | Logistic regression | LASSO | Random forest | XGBoost |
|---|---|---|---|---|
| AUROC | 0.78 (0.74–0.82) | 0.83 (0.79–0.87) | 0.82 (0.78–0.86) | 0.75 (0.70–0.80) |
| PRAUC | 0.35 (0.29–0.41) | 0.42 (0.38–0.46) | 0.48 (0.42–0.54) | 0.37 (0.26–0.44) |
| Accuracy | 0.88 | 0.90 | 0.92 | 0.87 |
| Cohen’s kappa | 0.21 | 0.36 | 0.52 | 0.28 |
| Sensitivity | 0.36 | 0.36 | 0.50 | 0.36 |
| Specificity | 0.93 | 0.96 | 0.97 | 0.93 |
| Precision | 0.38 | 0.50 | 0.64 | 0.37 |
| F1 score | 0.37 | 0.42 | 0.56 | 0.36 |
| Brier score | 0.08 | 0.07 | 0.07 | 0.08 |
Evaluation of the performance of logistic regression, LASSO, random forest, and XGBoost.
AUC, area under the curve; LASSO, least absolute shrinkage and selection operator; PRAUC, precision-recall area under the curve; ROC, receiver operating characteristic; XGBoost, extreme gradient boosting.
Model performance compared with traditional methods and existing score
The ML model showed a better accuracy to predict in-hospital MAE compared with a logistic regression model in the testing cohort [AUROC: 0.82, 95% CI (0.78–0.86) vs. 0.78, 95% CI (0.74–0.82); PRAUC: 0.48, 95% CI (0.42–0.54) vs. 0.35, 95% CI (0.29–0.41); Figure 3]. The proposed ML model exhibited the best performance for the prediction of in-hospital MAE compared with the ACUTE HF score,17 with a higher AUROC (0.82 vs. 0.57) and PRAUC (0.48 vs. 0.12; Figure 4). The performances of the ML model were globally similar in women and men (see Supplementary material online, Supplement S6). As sensitivity analysis, our ML model exhibited the best performance for the prediction of in-hospital all-cause death compared with traditional statistical method with a higher AUROC (0.81 vs. 0.76) and PRAUC (0.46 vs. 0.32; Supplementary material online, Supplement S7).
Figure 4.
Comparison of our model with ACUTE HF score. Receiver operating characteristic (area under the receiver operating curve, A) and precision-recall curves (precision-recall area under the curve, B) for prediction of in-hospital major adverse event were used to compare our machine learning model to the ACUTE HF score. AUC, area under the curve; PR, precision-recall; ROC, receiver operating curve.
Calibration of our machine learning model
The Hosmer–Lemeshow goodness-of-fit test showed no significant difference between the observed and predicted proportions of in-hospital MAE (P = 0.91) in the testing cohort, in line with the Brier score of 0.07. All these findings indicated a good calibration of the proposed ML model across all risk levels (see Supplementary material online, Supplement S8).
Discussion
In a prospective multicentre cohort study of consecutive patients hospitalized for AHF in ICCU, we built a ML model to predict in-hospital outcomes (Graphical Abstract). This ML model was based on few simple clinical, environmental, and echocardiographic variables: mean arterial pressure, ischaemic AHF aetiology, recreational drug use, exhaled CO level, LVOT VTI, peak E/e′ ratio, and TAPSE. The main findings are as follows: (i) the ML model exhibited a better performance than traditional statistical methods; (ii) the ML model outperformed existing scores as ACUTE HF score; (iii) its calibration was excellent; and (iv) among all the variables available, the feature selection using ML highlighted the importance of environmental data as recreational drug use and exhaled CO level. The study presents the first large-scale investigation of ML for prognostic risk evaluation using a wide spectrum of data ranging from clinical to echocardiography through environmental data. These findings suggest that ML and environmental factors could have an important role for risk stratification and decision-making in patients with AHF.
In line with prior large cohorts with a rate of in-hospital outcomes between 5 and 10%,2,18,19 we reported a rate of in-hospital MAE of 10.2% and in-hospital death of 1.7%. In our cohort, MAE was more driven by cardiogenic shock than all-cause in-hospital mortality and resuscitated cardiac arrest events.
Regarding the environmental factors selected by ML, exhaled CO level was one of the highest ranked features for risk prediction. It has been previously described as a measure of particular interest for the study of active and passive smoking exposure as an important marker of air pollution. An ancillary study of patients from the Framingham cohort showed an association between exhaled CO level and the occurrence of cardiovascular outcomes regardless of the patient's smoking status.20 This suggests that exhaled CO level could be an interesting marker for assessing the cardiovascular impact of air pollution. Indeed, an important meta-analysis showed a strong association between daily increases in gaseous air pollutants (particulate matter, CO, sulfur dioxide, nitrogen dioxide, and ozone) and the occurrence of severe HF outcomes.9 Although more studies from developing nations are required, air pollution is a pervasive public health issue with major cardiovascular and health economic burden, and it should remain a key target for global health policy. In line with these results, our working group has very recently shown that elevated CO level is independently associated with a 10-fold increase in in-hospital MAE and six-fold 1-year mortality in patients hospitalized for acute cardiac events.8 All these findings are also supported by Tun et al.,21 who showed that increased levels of exhaled CO were associated with an adverse cardiovascular biomarker profile and a higher risk of AHF, especially with reduced LVEF.
The second environmental factor selected by ML was recreational drug use. This finding is in line with a prior analysis from ADDICT-ICCU study showing that recreational drugs use is strongly associated with the occurrence of in-hospital outcomes with an incremental prognostic value above traditional risk factors.6 These findings can be explained by several types of sympathomimetic effects of recreational drugs, which can increase heart rate, blood pressure, temperature, and consequently myocardial oxygen demand.22
Two clinical variables were selected by ML with mean blood pressure and ischaemic aetiology of HF. Blood pressure is a well-known feature present in most of AHF scores.23,24 In the current study, we preferred the mean blood pressure than systolic blood pressure because it includes the effect of the diastolic value, which can reflect different situations as the aortic regurgitation or vasoplegia. Knowing that ischaemic aetiology is also well described as prognosticator for HF outcomes, two scores already published have also selected this feature as a prognosis factor.19,25
Beyond the traditional clinical data, the current study emphasized the importance of echocardiographic parameters at baseline in ICCU for risk stratification in AHF patients. Indeed, among the seven parameters selected by ML, three were echocardiographic variables with LVOT VTI, peak E/e′ ratio, and TAPSE, which are mean full in the day-to-day practice and can be performed by any cardiologist. Several studies have shown that left ventricular dysfunction with a drop in cardiac output,3,4 assessed by the LVOT VTI, was associated with a poor prognosis in a wide spectrum of acute cardiovascular diseases. Right ventricular dysfunction defined using TAPSE26,27 and left ventricular filling pressure assessed by the peak E/e′ ratio have also been described as two strong prognosticators in the acute setting of AHF.2,3,28 Consistently, these three echocardiographic parameters have a central place in the last European Society of Cardiology guidelines.3,4
Finally, our ML model showed the best accuracy to predict in-hospital outcomes compared with traditional methods and existing score assessed of AHF patients in ICCU. In particular, its performance outstripped ACUTE HF score.17 Up to date, no specific ML model including clinical, biological, environmental, and echocardiographic data has been built.
Study limitations
First, the sample size of patients analysed was decreased due to the split between training and testing cohorts. In addition, the exclusion of eight centres to build an external validation cohort also lowered the sample size. Nevertheless, as there are no other studies on the AHF population that have measured exhaled CO and recreational drug use, the results necessarily had to be validated on a part of the ADDICT-ICCU study. This lack of external validation from another country is an important limitation of the application of these findings, and we need to have further studies to validate them in other countries. Second, even if recreational drugs use6 and high exhaled CO level have recently been reported as increasing cardiovascular events8 and had pathophysiological background on cardiovascular system, their screenings are not available in daily practice, hence a limited translation in practice. Indeed, exhaled CO level and recreational drug detection are not routinely assessed in all centres worldwide. Even if the echocardiographic findings of this study are important, we must acknowledge that a complete echocardiography including LVEF as collected in this study within 24 h after admission in ICCU is not systematically performed in clinical routine. Third, missing values always represent a challenge in medical research. In line with the literature,29 we imputed missing data using with K-Nearest Neighbor algorithm for all data with a missing data rate < 40%. Fourth, some ICCU scores could not be calculated due to missing biological collected data. The only score we could compare with was ACUTE HF score,17 and results may be interpreted with caution. The SCAI shock score was not available in this study. Finally, this study assessed only the performance of ML, but not its practical implementation. Additional parameters would have been of interest, especially renal function (not only creatininemia, C-reactive protein, intake of some drugs…). Once validated in clinical routine, further randomized clinical trials based upon ML risk stratification using environmental data should be considered.
Conclusion
In a large multicentre registry of consecutive patients hospitalized for AHF in ICCU, a ML model using seven clinical, environmental—including exhaled CO level and recreational drugs use—and echocardiographic variables was an accurate method for predicting in-hospital MAE. This ML model exhibited a higher prognostic value to predict in-hospital MAE than all statistical methods or existing score.
Lead author biography

Dr Benjamin Sibilia is a cardiologist at Lariboisière-Saint Louis Hospital, Paris, since 2023, and is affiliated with the University of Paris-Cité. He holds a Master’s degree in Methodology and Statistics in Medical Research. His clinical and research interests focus on heart failure, cardiovascular prevention, and cardio-oncology. He is actively involved in the MIRACL.ai platform (Multimodality Imaging for Research and Analysis Core Laboratory and Artificial Intelligence, AP-HP, Paris, France), a cutting-edge platform that integrates academic research, artificial intelligence with machine learning projects, and industry to advance diagnostic and prognostic tools in cardiology.
Supplementary Material
Acknowledgements
We thank the medical, paramedical, and research staff of all investigating centres involved.
Contributor Information
Benjamin Sibilia, Service de Cardiologie, Université Paris Cité, Hôpital Lariboisière, Assistance Publique-Hôpitaux de Paris, 2 rue Ambroise Paré, Paris 75010, France; Inserm MASCOT—UMRS 942, University Hospital of Lariboisiere, 2 rue Ambroise Paré, Paris 75010, France; MIRACL.ai Laboratory, Multimodality Imaging for Research and Analysis Core Laboratory and Artificial Intelligence, University Hospital of Lariboisiere (AP-HP), 2 rue Ambroise Paré, Paris 75010, France.
Solenn Toupin, Service de Cardiologie, Université Paris Cité, Hôpital Lariboisière, Assistance Publique-Hôpitaux de Paris, 2 rue Ambroise Paré, Paris 75010, France; Inserm MASCOT—UMRS 942, University Hospital of Lariboisiere, 2 rue Ambroise Paré, Paris 75010, France; MIRACL.ai Laboratory, Multimodality Imaging for Research and Analysis Core Laboratory and Artificial Intelligence, University Hospital of Lariboisiere (AP-HP), 2 rue Ambroise Paré, Paris 75010, France.
Nabil Bouali, Department of Cardiology, University Hospital of Poitiers, Poitiers 86000, France; Service de Cardiologie, Centre Hospitalier de Saintonge, 11, Boulevard Ambroise-Paré, Saintes 17100, France.
Jean-Baptiste Brette, Cardiology Department, Rangueil University Hospital, Toulouse, France.
Arthur Ramonatxo, Department of Cardiology, University Hospital of Poitiers, Poitiers 86000, France.
Guillaume Schurtz, Department of Cardiology, University Hospital of Lille, Lille, France.
Kenza Hamzi, Service de Cardiologie, Université Paris Cité, Hôpital Lariboisière, Assistance Publique-Hôpitaux de Paris, 2 rue Ambroise Paré, Paris 75010, France; Inserm MASCOT—UMRS 942, University Hospital of Lariboisiere, 2 rue Ambroise Paré, Paris 75010, France; MIRACL.ai Laboratory, Multimodality Imaging for Research and Analysis Core Laboratory and Artificial Intelligence, University Hospital of Lariboisiere (AP-HP), 2 rue Ambroise Paré, Paris 75010, France.
Antonin Trimaille, Department of Cardiovascular Medicine, Nouvel Hôpital Civil, Strasbourg University Hospital, Strasbourg 67000, France.
Emmanuel Gall, Service de Cardiologie, Université Paris Cité, Hôpital Lariboisière, Assistance Publique-Hôpitaux de Paris, 2 rue Ambroise Paré, Paris 75010, France; Inserm MASCOT—UMRS 942, University Hospital of Lariboisiere, 2 rue Ambroise Paré, Paris 75010, France; MIRACL.ai Laboratory, Multimodality Imaging for Research and Analysis Core Laboratory and Artificial Intelligence, University Hospital of Lariboisiere (AP-HP), 2 rue Ambroise Paré, Paris 75010, France.
Nicolas Piliero, Service de Cardiologie, Univ. Grenoble Alpes, CHU Grenoble Alpes, Grenoble 38000, France.
Alexandre Unger, Service de Cardiologie, Université Paris Cité, Hôpital Lariboisière, Assistance Publique-Hôpitaux de Paris, 2 rue Ambroise Paré, Paris 75010, France; Inserm MASCOT—UMRS 942, University Hospital of Lariboisiere, 2 rue Ambroise Paré, Paris 75010, France; MIRACL.ai Laboratory, Multimodality Imaging for Research and Analysis Core Laboratory and Artificial Intelligence, University Hospital of Lariboisiere (AP-HP), 2 rue Ambroise Paré, Paris 75010, France; Department of Cardiology, Hôpital Universitaire de Bruxelles, Hôpital Erasme, Université Libre de Bruxelles, Brussels, Belgium.
Stéphane Andrieu, Service de Cardiologie, Hôpital Henri Duffaut, Avignon 84902, France.
Trecy Gonçalves, Service de Cardiologie, Université Paris Cité, Hôpital Lariboisière, Assistance Publique-Hôpitaux de Paris, 2 rue Ambroise Paré, Paris 75010, France; Inserm MASCOT—UMRS 942, University Hospital of Lariboisiere, 2 rue Ambroise Paré, Paris 75010, France; MIRACL.ai Laboratory, Multimodality Imaging for Research and Analysis Core Laboratory and Artificial Intelligence, University Hospital of Lariboisiere (AP-HP), 2 rue Ambroise Paré, Paris 75010, France.
Fabien Picard, Service de Cardiologie, Université Paris Cité, Hôpital Cochin, Paris, France.
Vincent Roule, Department of Cardiology, Caen University Hospital, Caen, France.
François Roubille, Cardiology Department, University Hospital, PhyMedExp, University of Montpellier, INSERM, CNRS, CHRU, INI-CRT, Montpellier, France.
Sonia Houssany-Pissot, Service de Cardiologie et Médecine Aéronautique, Hôpital d'Instruction des Armées Percy, Clamart 92140, France.
Océane Bouchot, Service de Cardiologie, Centre Hospitalier Annecy Genevois, Epagny Metz-Tessy 74370, France.
Victor Aboyans, Department of Cardiology, University Hospital of Limoges, Limoges, France.
Reza Rossanaly Vasram, Department of Cardiology, Felix-Guyon University Hospital, Saint-Denis-de-La-Reunion, France.
Thomas Bochaton, Intensive Cardiological Care Division, Louis Pradel Hospital, Hospices Civils de Lyon, Bron, France.
Damien Logeart, Service de Cardiologie, Université Paris Cité, Hôpital Lariboisière, Assistance Publique-Hôpitaux de Paris, 2 rue Ambroise Paré, Paris 75010, France; Inserm MASCOT—UMRS 942, University Hospital of Lariboisiere, 2 rue Ambroise Paré, Paris 75010, France.
Alain Cohen Solal, Service de Cardiologie, Université Paris Cité, Hôpital Lariboisière, Assistance Publique-Hôpitaux de Paris, 2 rue Ambroise Paré, Paris 75010, France; Inserm MASCOT—UMRS 942, University Hospital of Lariboisiere, 2 rue Ambroise Paré, Paris 75010, France.
Jérôme Cartailler, Inserm MASCOT—UMRS 942, University Hospital of Lariboisiere, 2 rue Ambroise Paré, Paris 75010, France; MIRACL.ai Laboratory, Multimodality Imaging for Research and Analysis Core Laboratory and Artificial Intelligence, University Hospital of Lariboisiere (AP-HP), 2 rue Ambroise Paré, Paris 75010, France.
Alexandre Mebazaa, Inserm MASCOT—UMRS 942, University Hospital of Lariboisiere, 2 rue Ambroise Paré, Paris 75010, France.
Jean-Guillaume Dillinger, Service de Cardiologie, Université Paris Cité, Hôpital Lariboisière, Assistance Publique-Hôpitaux de Paris, 2 rue Ambroise Paré, Paris 75010, France; Inserm MASCOT—UMRS 942, University Hospital of Lariboisiere, 2 rue Ambroise Paré, Paris 75010, France; MIRACL.ai Laboratory, Multimodality Imaging for Research and Analysis Core Laboratory and Artificial Intelligence, University Hospital of Lariboisiere (AP-HP), 2 rue Ambroise Paré, Paris 75010, France.
Patrick Henry, Service de Cardiologie, Université Paris Cité, Hôpital Lariboisière, Assistance Publique-Hôpitaux de Paris, 2 rue Ambroise Paré, Paris 75010, France; Inserm MASCOT—UMRS 942, University Hospital of Lariboisiere, 2 rue Ambroise Paré, Paris 75010, France; MIRACL.ai Laboratory, Multimodality Imaging for Research and Analysis Core Laboratory and Artificial Intelligence, University Hospital of Lariboisiere (AP-HP), 2 rue Ambroise Paré, Paris 75010, France.
Théo Pezel, Service de Cardiologie, Université Paris Cité, Hôpital Lariboisière, Assistance Publique-Hôpitaux de Paris, 2 rue Ambroise Paré, Paris 75010, France; Inserm MASCOT—UMRS 942, University Hospital of Lariboisiere, 2 rue Ambroise Paré, Paris 75010, France; MIRACL.ai Laboratory, Multimodality Imaging for Research and Analysis Core Laboratory and Artificial Intelligence, University Hospital of Lariboisiere (AP-HP), 2 rue Ambroise Paré, Paris 75010, France.
Supplementary material
Supplementary material is available at European Heart Journal – Digital Health.
Funding
This research received an institutional grant from the French Heart Foundation, “Fondation Coeur et Recherche” (Paris, France).
Data availability
Data are available upon reasonable request.
References
- 1. Crespo-Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail 2016;18:613–625. [DOI] [PubMed] [Google Scholar]
- 2. Lombardi C, Peveri G, Cani D, Latta F, Bonelli A, Tomasoni D, et al. In-hospital and long-term mortality for acute heart failure: analysis at the time of admission to the emergency department. ESC Heart Fail 2020;7:2650–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 4. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2023;44:3627–3639. [DOI] [PubMed] [Google Scholar]
- 5. Nishimura M, Bhatia H, Ma J, Dickson SD, Alshawabkeh L, Adler E, et al. The impact of substance abuse on heart failure hospitalizations. Am J Med 2020;133:207–213.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pezel T, Dillinger JG, Trimaille A, Delmas C, Piliero N, Bouleti C, et al. Prevalence and impact of recreational drug use in patients with acute cardiovascular events. Heart 2023;109:1608–1616. [DOI] [PubMed] [Google Scholar]
- 7. Curran L, Nah G, Marcus GM, Tseng Z, Crawford MH, Parikh NI. Clinical correlates and outcomes of methamphetamine-associated cardiovascular diseases in hospitalized patients in California. J Am Heart Assoc 2022;11:e023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dillinger JG, Pezel T, Delmas C, Schurtz G, Trimaille A, Piliero N, et al. Carbon monoxide and prognosis in smokers hospitalised with acute cardiac events: a multicentre, prospective cohort study. eClinicalMedicine 2024;67:102401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shah AS, Langrish JP, Nair H, McAllister DA, Hunter AL, Donaldson K, et al. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet 2013;382:1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mark E, Goldsman D, Gurbaxani B, Keskinocak P, Sokol J. Using machine learning and an ensemble of methods to predict kidney transplant survival. PLoS One 2019;14:e0209068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pezel T, Sanguineti F, Garot P, Unterseeh T, Champagne S, Toupin S, et al. Machine-learning score using stress CMR for death prediction in patients with suspected or known CAD. JACC Cardiovasc Imaging 2022;15:1900–1913. [DOI] [PubMed] [Google Scholar]
- 12. Dillinger JG, Pezel T, Fauvel C, Delmas C, Schurtz G, Trimaille A, et al. Prevalence of psychoactive drug use in patients hospitalized for acute cardiac events: rationale and design of the ADDICT-ICCU trial, from the emergency and acute cardiovascular care working group and the National College of Cardiologists in training of the French Society of Cardiology. Arch Cardiovasc Dis 2022;115:514–520. [DOI] [PubMed] [Google Scholar]
- 13. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (writing committee to develop cardiovascular endpoints data standards). J Am Coll Cardiol 2015;66:403–469. [DOI] [PubMed] [Google Scholar]
- 14. Sengupta PP, Shrestha S, Berthon B, Messas E, Donal E, Tison GH, et al. Proposed Requirements for Cardiovascular Imaging-Related Machine Learning Evaluation (PRIME): a checklist: reviewed by the American College of Cardiology healthcare innovation council. JACC Cardiovasc Imaging 2020;13:2017–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B Stat Methodol 1996;58:267–288. [Google Scholar]
- 16. Krstajic D, Buturovic LJ, Leahy DE, Thomas S. Cross-validation pitfalls when selecting and assessing regression and classification models. J Cheminform 2014 29;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cameli M, Pastore MC, Carli GD, Henein MY, Mandoli GE, Lisi E, et al. ACUTE HF score, a multiparametric prognostic tool for acute heart failure: a real-life study. Int J Cardiol 2019;296:103–108. [DOI] [PubMed] [Google Scholar]
- 18. Abraham WT, Fonarow GC, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). J Am Coll Cardiol 2008;52:347–356. [DOI] [PubMed] [Google Scholar]
- 19. Miró Ò, Rossello X, Gil V, Martín-Sánchez FJ, Llorens P, Herrero-Puente P, et al. Predicting 30-day mortality for patients with acute heart failure in the emergency department: a cohort study. Ann Intern Med 2017;167:698–705. [DOI] [PubMed] [Google Scholar]
- 20. Cheng S, Enserro D, Xanthakis V, Sullivan LM, Murabito JM, Benjamin EJ, et al. Association of exhaled carbon monoxide with subclinical cardiovascular disease and their conjoint impact on the incidence of cardiovascular outcomes. Eur Heart J 2014;35:2980–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tun B, Ehrbar R, Short M, Cheng S, Vasan RS, Xanthakis V. Association of exhaled carbon monoxide with ideal cardiovascular health, circulating biomarkers, and incidence of heart failure in the Framingham Offspring Study. J Am Heart Assoc 2020;9:e016762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghuran A, Van Der Wieken LR, Nolan J. Cardiovascular complications of recreational drugs. BMJ 2001;323:464–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee DS, Stitt A, Austin PC, Stukel TA, Schull MJ, Chong A, et al. Prediction of heart failure mortality in emergent care. Ann Intern Med 2012;156:767–775. [DOI] [PubMed] [Google Scholar]
- 24. Peterson PN, Rumsfeld JS, Liang L, Albert NM, Hernandez AF, Peterson ED, et al. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association Get With the Guidelines program. Circ Cardiovasc Qual Outcomes 2010;3:25–32. [DOI] [PubMed] [Google Scholar]
- 25. Stiell IG, Clement CM, Brison RJ, Rowe BH, Borgundvaag B, Aaron SD, et al. A risk scoring system to identify emergency department patients with heart failure at high risk for serious adverse events. Acad Emerg Med 2013;20:17–26. [DOI] [PubMed] [Google Scholar]
- 26. Zehender M, Kasper W, Kauder E, Schönthaler M, Geibel A, Olschewski M, et al. Right ventricular infarction as an independent predictor of prognosis after acute inferior myocardial infarction. N Engl J Med 1993;328:981–988. [DOI] [PubMed] [Google Scholar]
- 27. Berrill M, Ashcroft E, Fluck D, John I, Beeton I, Sharma P, et al. Right ventricular dysfunction predicts outcome in acute heart failure. Front Cardiovasc Med 2022;9:911053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jentzer JC, Anavekar NS, Bennett C, Murphree DH, Keegan MT, Wiley B, et al. Derivation and validation of a novel cardiac intensive care unit admission risk score for mortality. J Am Heart Assoc 2019;8:e013675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials—a practical guide with flowcharts. BMC Med Res Methodol 2017;17:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.