Abstract
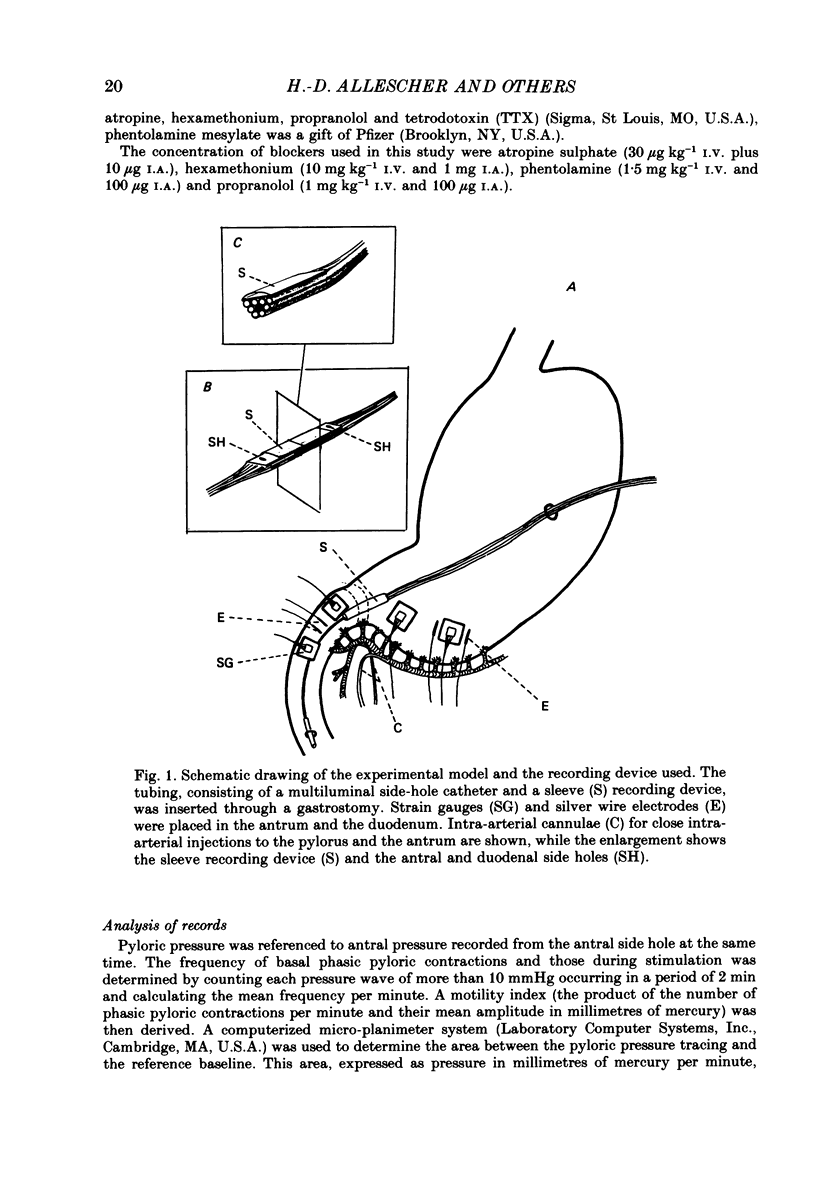
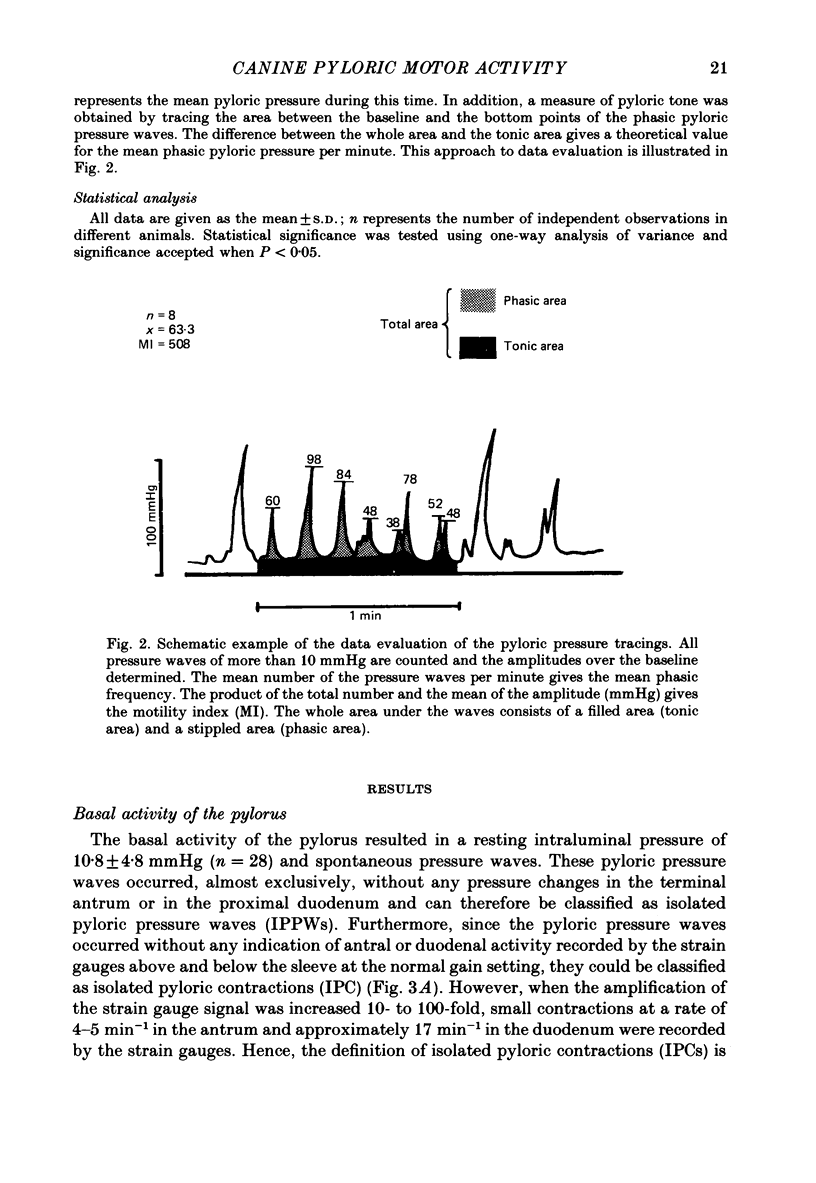
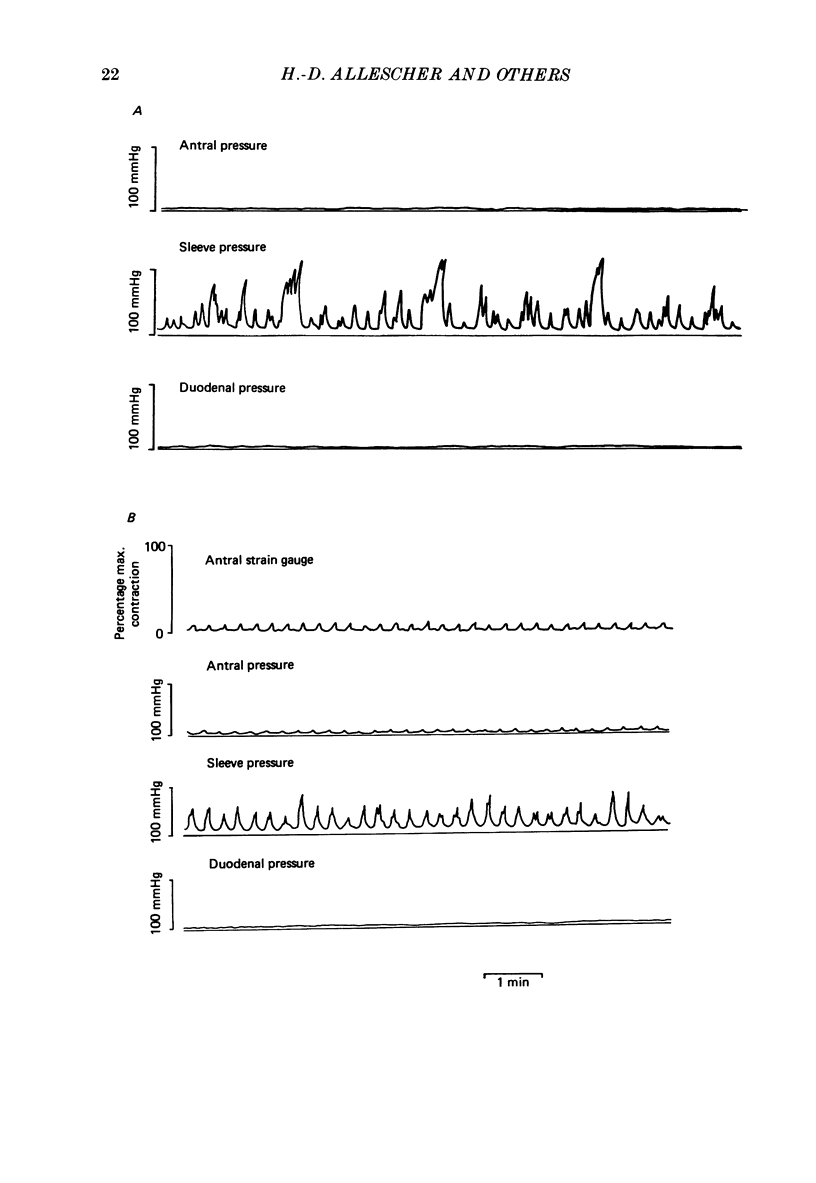
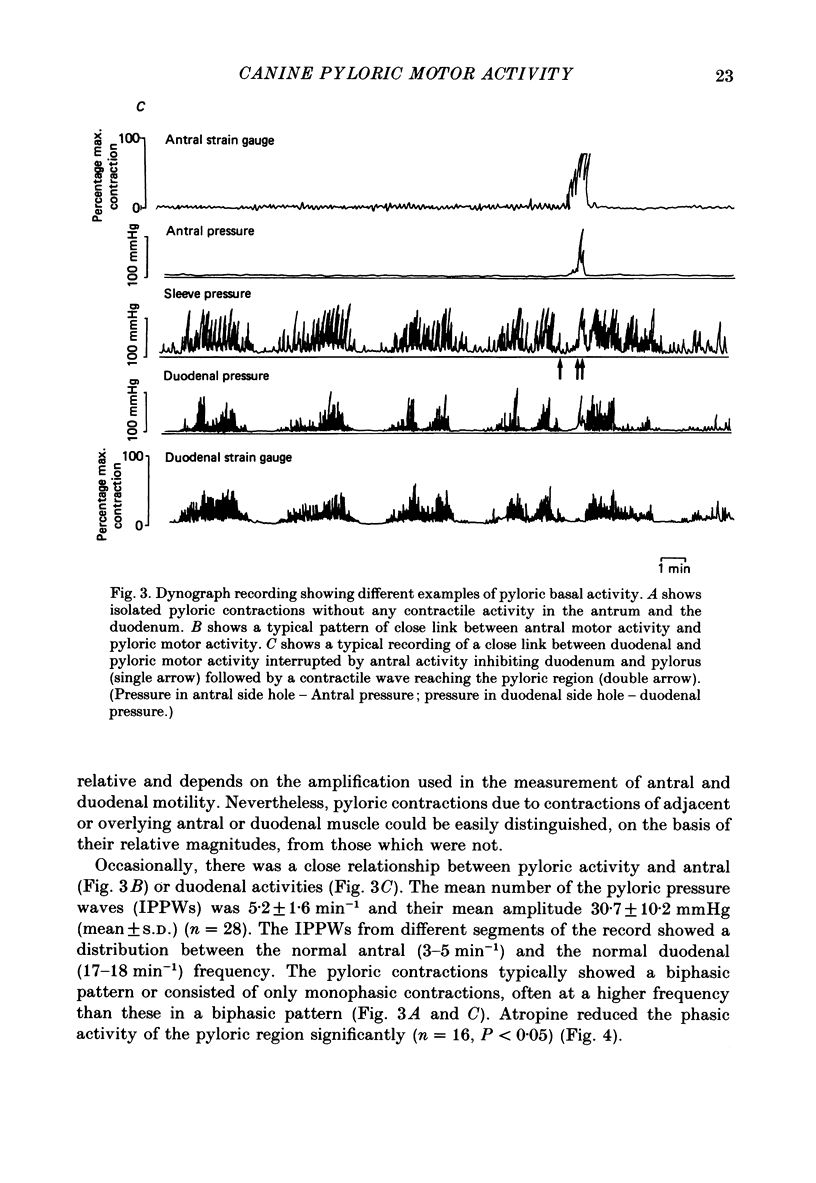
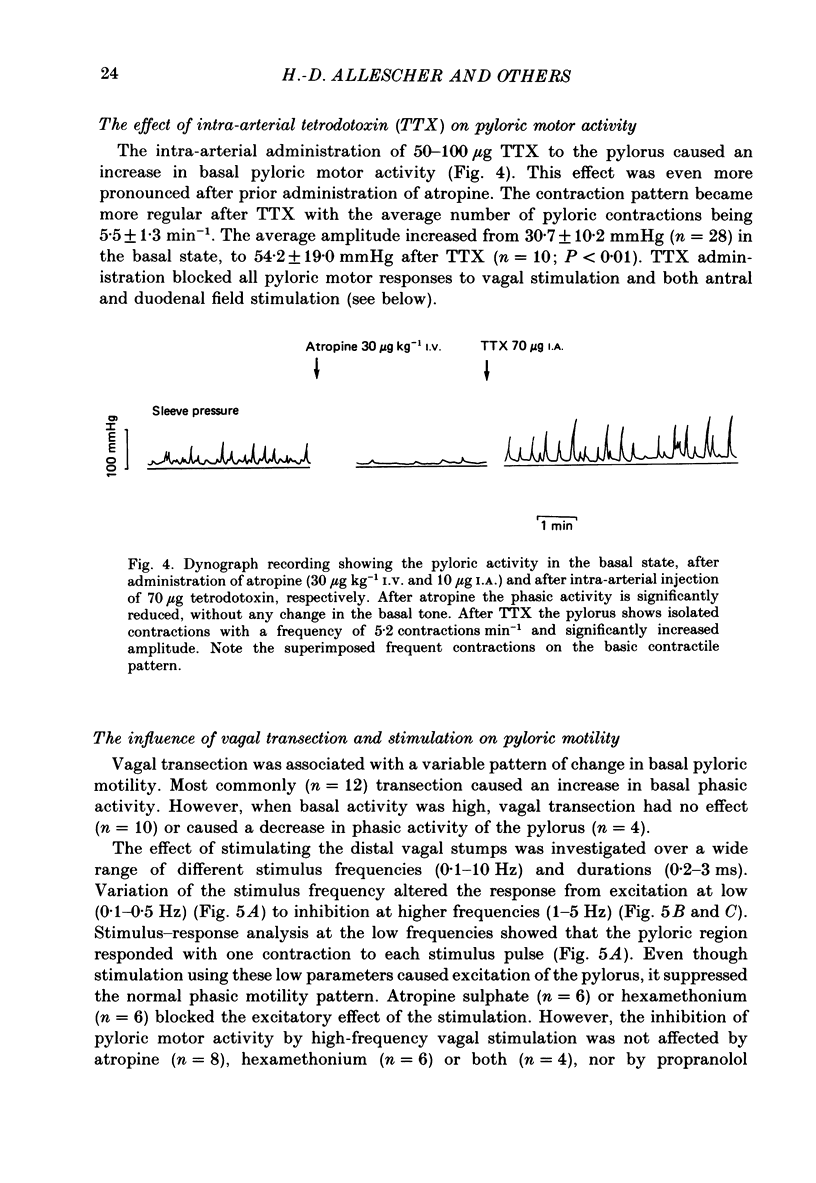
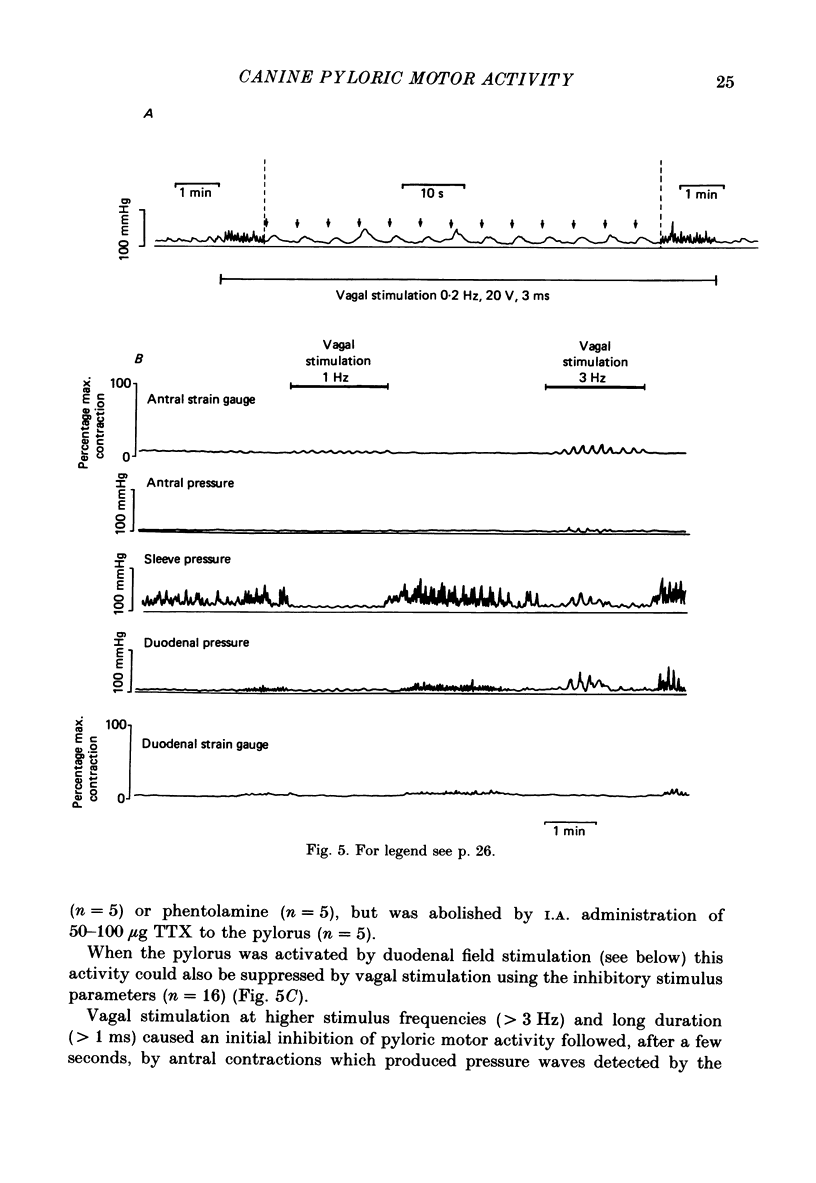
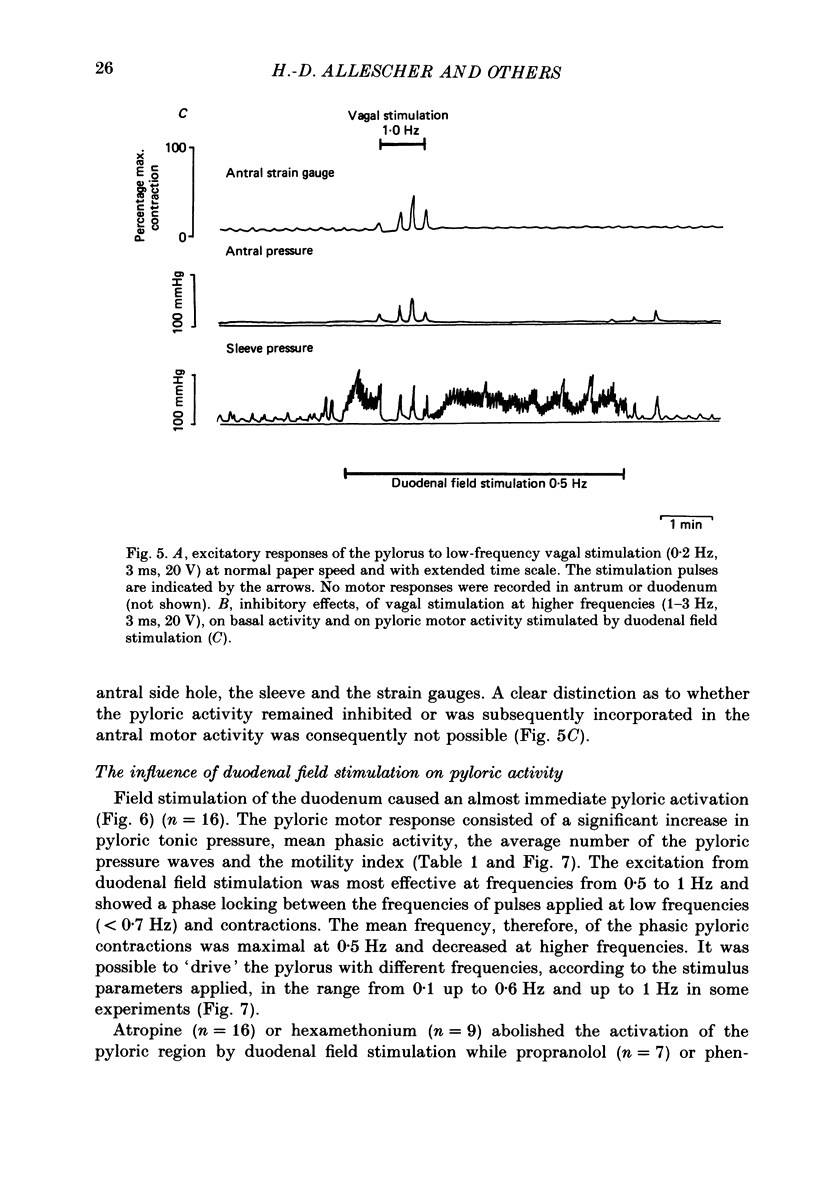
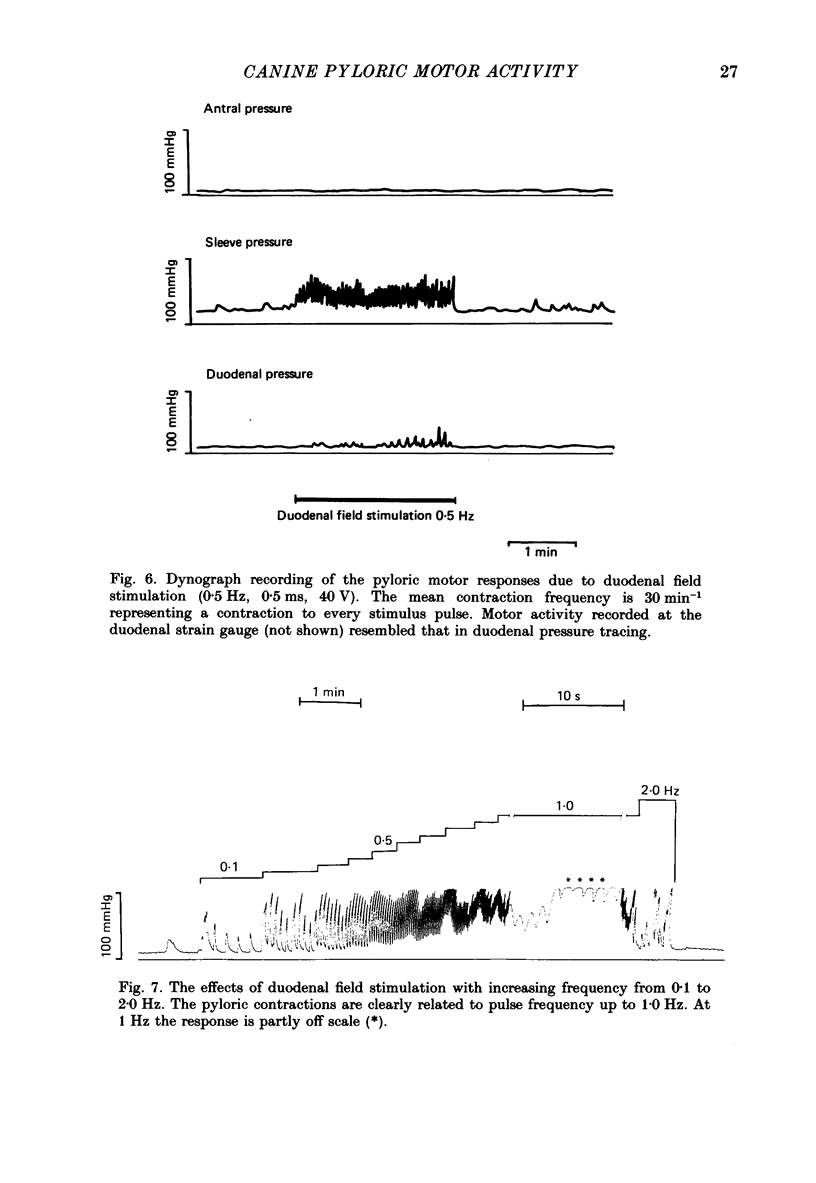
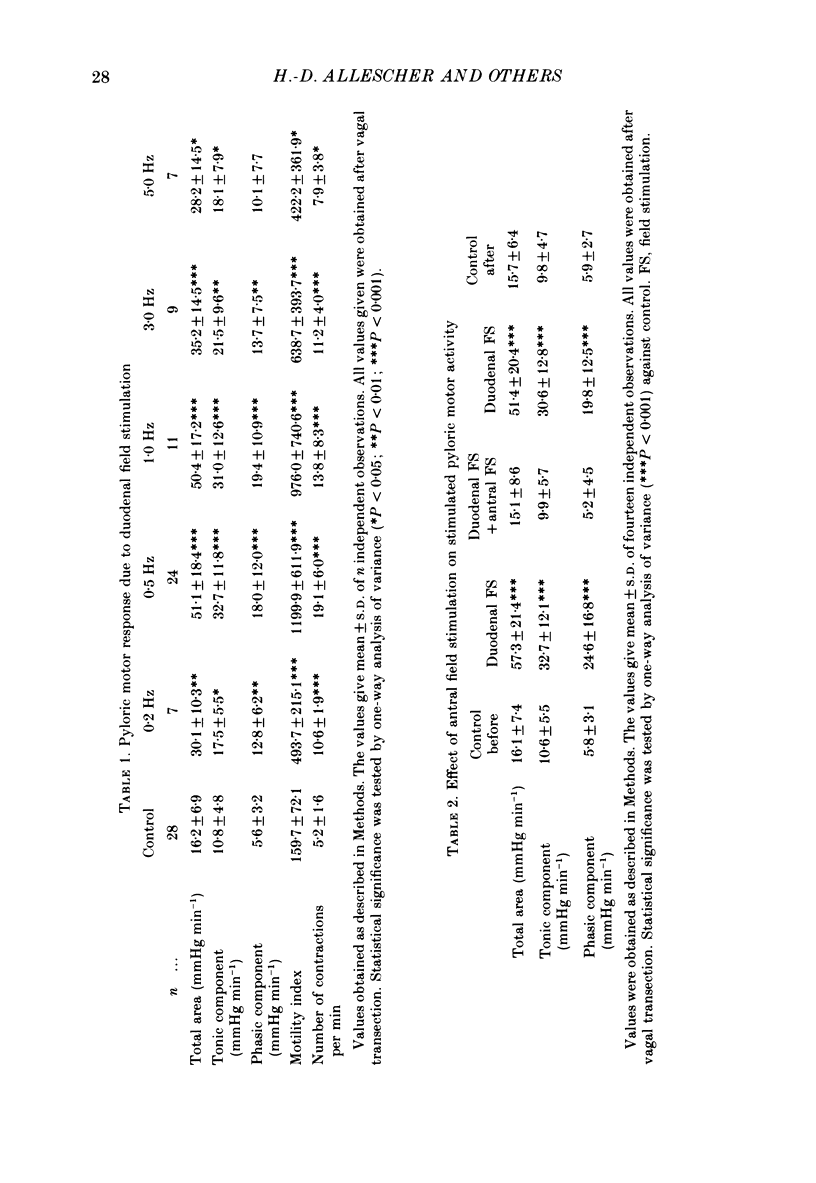
1. In chloralose-urethane-anaesthetized dogs a manometric assembly was inserted via a gastrostomy to monitor pyloric pressure with a sleeve sensor. Antral and duodenal contractions were monitored with both manometric side holes and serosal strain gauges. 2. Subserosal silver wire electrodes were placed in the antrum 5 cm orad and the duodenum 3 cm aborad to the pylorus to facilitate field stimulation of intramural nerves. 3. The pylorus exerted spontaneous tone (10.8 +/- 4.8 mmHg) with phasic contractions occurring at a rate varying from 1-5 min-1 and, at times, with a superimposed higher frequency up to 15 min-1. Atropine (30 micrograms kg-1 I.V. and 10 micrograms I.A.) reduced and tetrodotoxin (50-100 micrograms I.A.) enhanced the phasic activity significantly. 4. Bilateral cervical vagal section had no consistent influence on pyloric motility. 5. Stimulation of the distal ends of the cervical vagal nerves at low frequencies (0.2-0.5 Hz, 1-3 ms, 20 V) induced phasic pyloric contractions, which were abolished by atropine or hexamethonium (10 mg kg-1 I.V. and 1 mg I.A.). Higher frequencies (greater than 0.7 Hz) of stimulation inhibited both phasic and tonic contractions and this inhibition was unaffected by atropine, hexamethonium, phentolamine (1.5 mg kg-1 I.V. and 100 micrograms I.A.) or propranolol (1 mg kg-1 I.V. and 100 micrograms I.A.). All neural responses were blocked by tetrodotoxin (50-100 micrograms I.A.). 6. Duodenal field stimulation (0.2-5 Hz, 0.5 ms, 40 V) induced strong phasic and tonic contractions in the pylorus. This excitation was blocked by atropine, hexamethonium, tetrodotoxin (50-100 micrograms I.A.) or duodenal transection orad to the stimulating electrodes. 7. Antral field stimulation (0.5-1 Hz, 0.5 ms, 40 V) completely abolished phasic activity in the pylorus and reduced tonic activity, regardless of whether the contractile activity was spontaneous or induced by neural stimulation. This inhibitory action was unaffected by atropine, hexamethonium or propranolol but was blocked by tetrodotoxin and antral transection aborad to the stimulating electrodes. Phentolamine attenuated the inhibitory effect of antral field stimulation on pyloric motility. 8. It is concluded that the distal canine pylorus exhibits myogenic tone and phasic activity which is modulated by extrinsic and intrinsic nerve pathways. Vagal nerves contain fibres, activated by different stimulus parameters which can either excite or inhibit pyloric activity. Activation of antral nerves inhibits pyloric activity, with both non-adrenergic, non-cholinergic and phentolamine-sensitive pathways contributing to this inhibitory response.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
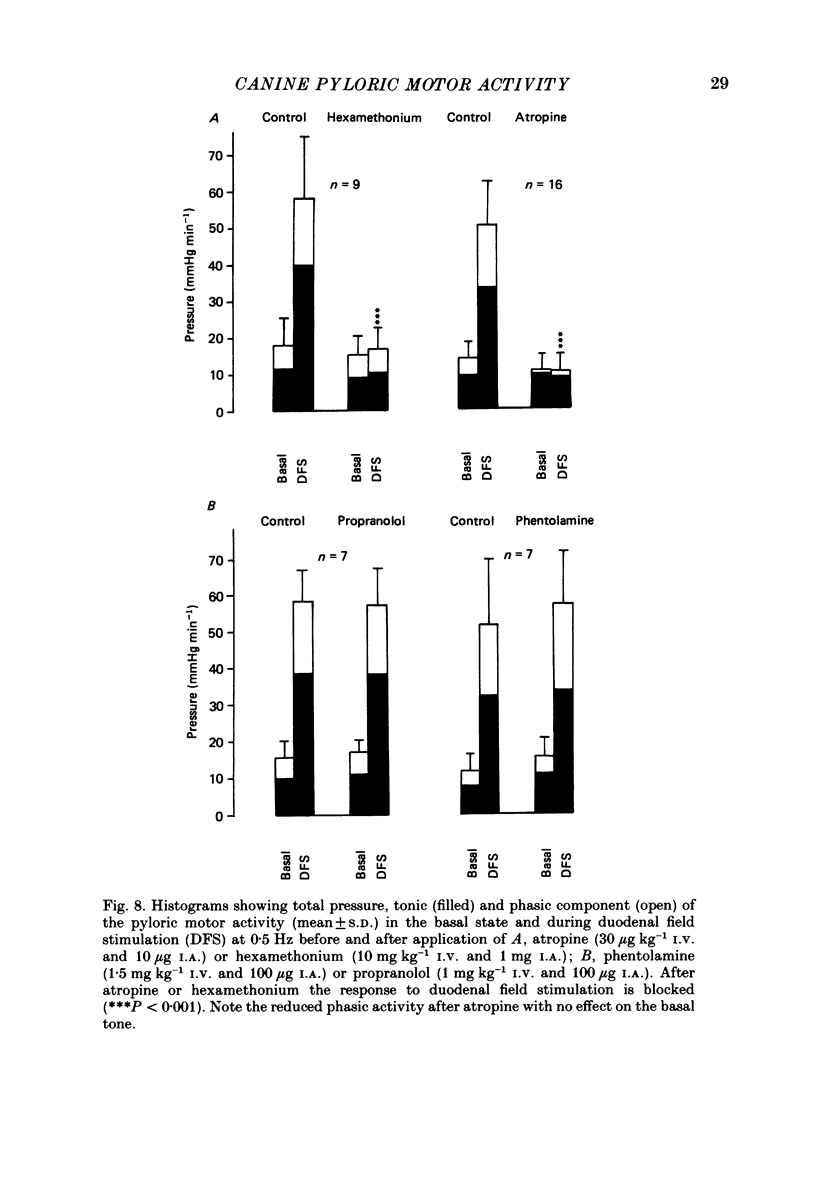
These references are in PubMed. This may not be the complete list of references from this article.
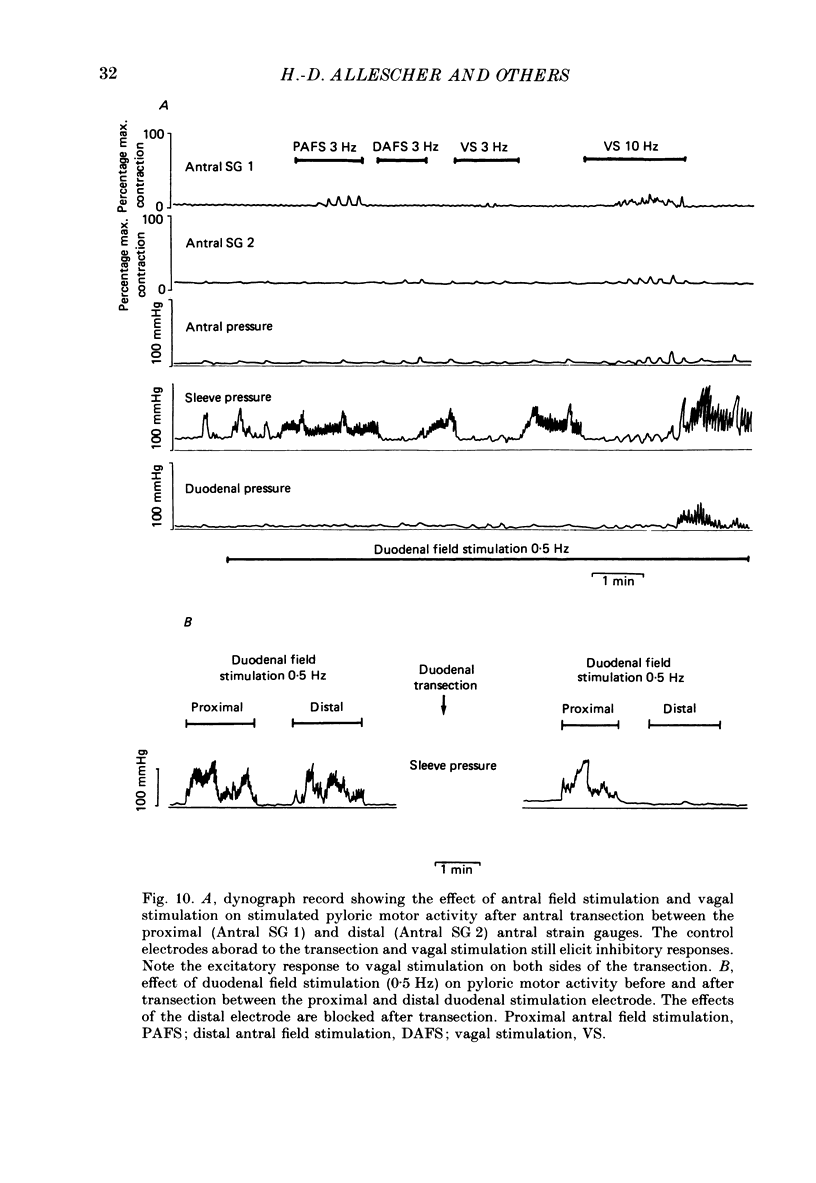
- Anuras S., Cooke A. R., Christensen J. An inhibitory innervation at the gastroduodenal junction. J Clin Invest. 1974 Sep;54(3):529–535. doi: 10.1172/JCI107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
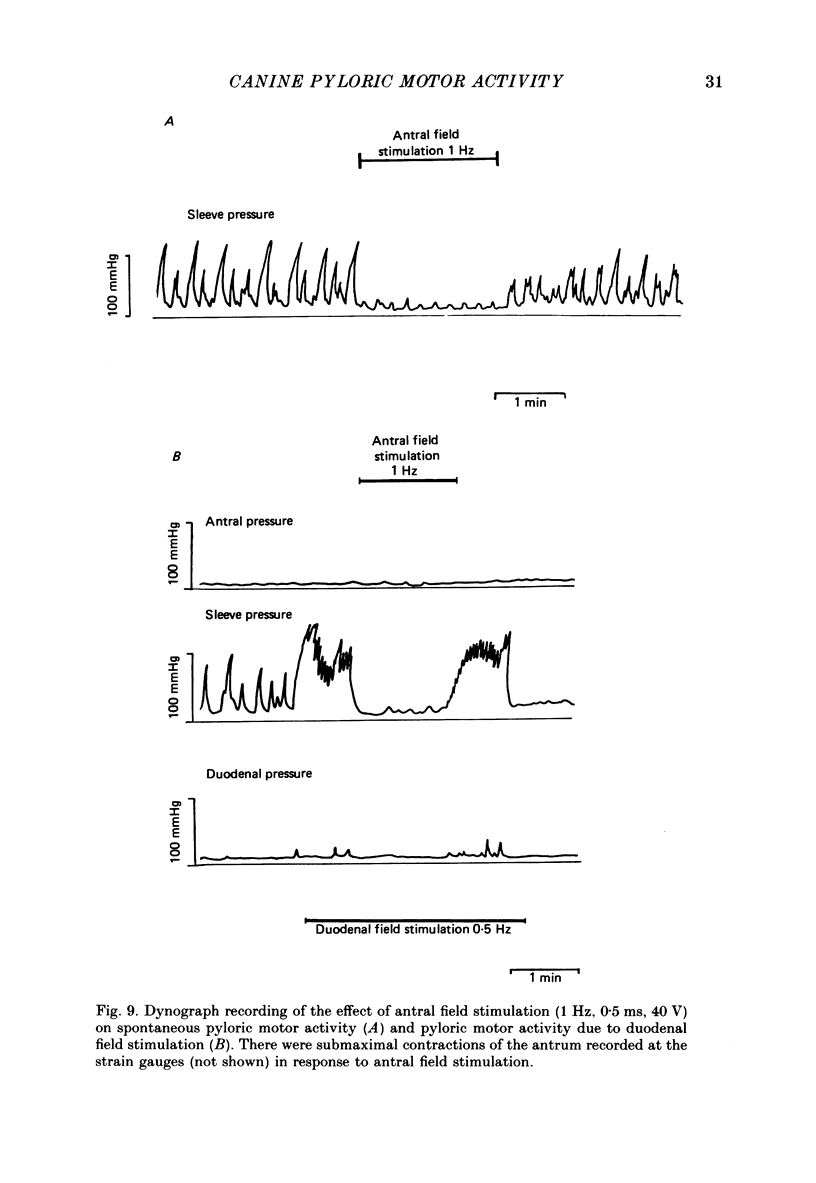
- BASS P., CODE C. F., LAMBERTEH Electric activity of gastroduodenal junction. Am J Physiol. 1961 Oct;201:587–592. doi: 10.1152/ajplegacy.1961.201.4.587. [DOI] [PubMed] [Google Scholar]
- BORTOFF A., WEG N. TRANSMISSION OF ELECTRICAL ACTIVITY THROUGH THE GASTRODUODENAL JUNCTION. Am J Physiol. 1965 Mar;208:531–536. doi: 10.1152/ajplegacy.1965.208.3.531. [DOI] [PubMed] [Google Scholar]
- BRINK B. M., SCHLEGEL J. F., CODE C. F. THE PRESSURE PROFILE OF THE GASTRODUODENAL JUNCTIONAL ZONE IN DOGS. Gut. 1965 Apr;6:163–171. doi: 10.1136/gut.6.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar J., Biancani P., Zabinski M. P. Characterization of feline gastroduodenal junction by neural and hormonal stimulation. Am J Physiol. 1979 Jan;236(1):E45–E51. doi: 10.1152/ajpendo.1979.236.1.E45. [DOI] [PubMed] [Google Scholar]
- Bortoff A., Davis R. S. Myogenic transmission of antral slow waves across the gastroduodenal junction in situ. Am J Physiol. 1968 Oct;215(4):889–897. doi: 10.1152/ajplegacy.1968.215.4.889. [DOI] [PubMed] [Google Scholar]
- Cai W. Q., Gabella G. Structure and innervation of the musculature at the gastroduodenal junction of the guinea-pig. J Anat. 1984 Aug;139(Pt 1):93–104. [PMC free article] [PubMed] [Google Scholar]
- Daniel E. E. The electrical and contractile activity of the pyloric region in dogs and the effects of drugs. Gastroenterology. 1965 Oct;49(4):403–418. [PubMed] [Google Scholar]
- Deloof S., Rousseau J. P. Specific effects of thoracic vagotomy on the electrical activity of the gastric antrum and pylorus in rabbits. Q J Exp Physiol. 1985 Oct;70(4):491–501. doi: 10.1113/expphysiol.1985.sp002936. [DOI] [PubMed] [Google Scholar]
- Dent J. A new technique for continuous sphincter pressure measurement. Gastroenterology. 1976 Aug;71(2):263–267. [PubMed] [Google Scholar]
- Fisher R., Cohen S. Physiological characteristics of the human pyloric sphincter. Gastroenterology. 1973 Jan;64(1):67–75. [PubMed] [Google Scholar]
- Langley J. N. On Inhibitory Fibres in the Vagus for the end of the OEsophagus and the Stomach. J Physiol. 1898 Dec 30;23(5):407–414. doi: 10.1113/jphysiol.1898.sp000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman S. H., Jacobowitz D. M., Mason G. R., Garber H. I., Ormsbee H. S., 3rd Gastric and pyloric motor response to sympathetic nerve stimulation after chemical sympathectomy. J Auton Nerv Syst. 1981 Sep;4(3):207–215. doi: 10.1016/0165-1838(81)90045-x. [DOI] [PubMed] [Google Scholar]
- Mearin F., Camilleri M., Malagelada J. R. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology. 1986 Jun;90(6):1919–1925. doi: 10.1016/0016-5085(86)90262-3. [DOI] [PubMed] [Google Scholar]
- Mir S. S., Telford G. L., Mason G. R., Ormsbee H. S., 3rd Noncholinergic nonadrenergic inhibitory innervation of the canine pylorus. Gastroenterology. 1979 Jun;76(6):1443–1448. [PubMed] [Google Scholar]
- Reynolds J. C., Ouyang A., Cohen S. Evidence for an opiate-mediated pyloric sphincter reflex. Am J Physiol. 1984 Feb;246(2 Pt 1):G130–G136. doi: 10.1152/ajpgi.1984.246.2.G130. [DOI] [PubMed] [Google Scholar]
- Sarna S. K., Daniel E. E. Electrical stimulation of gastric electrical control activity. Am J Physiol. 1973 Jul;225(1):125–131. doi: 10.1152/ajplegacy.1973.225.1.125. [DOI] [PubMed] [Google Scholar]
- Sarna S. K., Daniel E. E. Electrical stimulation of small intestinal electrical control activity. Gastroenterology. 1975 Sep;69(3):660–667. [PubMed] [Google Scholar]
- Schardt M., van der Zypen E. Enzymhistochemische und quantitative Untersuchungen über die regionalen Unterschiede des intramuralen Nervensystems im Magen-Darm-Kanal der weissen Laboratoriumsmaus. Acta Anat (Basel) 1974;90(3):403–430. [PubMed] [Google Scholar]
- Schulze-Delrieu K., Shirazi S. S. Neuromuscular differentiation of the human pylorus. Gastroenterology. 1983 Feb;84(2):287–292. [PubMed] [Google Scholar]
- Telford G. L., Mir S. S., Mason G. R., Ormsbee H. S., 3rd Neural control of the canine pylorus. Am J Surg. 1979 Jan;137(1):92–98. doi: 10.1016/0002-9610(79)90017-5. [DOI] [PubMed] [Google Scholar]
- Valenzuela J. E., Defilippi C., Csendes A. Manometric studies on the human pyloric sphincter. Effect of cigarette smoking, metoclopramide, and atropine. Gastroenterology. 1976 Apr;70(4):481–483. [PubMed] [Google Scholar]
- Weisbrodt N. W., Wiley J. N., Overholt B. F., Bass P. A relation between gastroduodenal muscle contractions and gastric empyting. Gut. 1969 Jul;10(7):543–548. doi: 10.1136/gut.10.7.543. [DOI] [PMC free article] [PubMed] [Google Scholar]


