Abstract
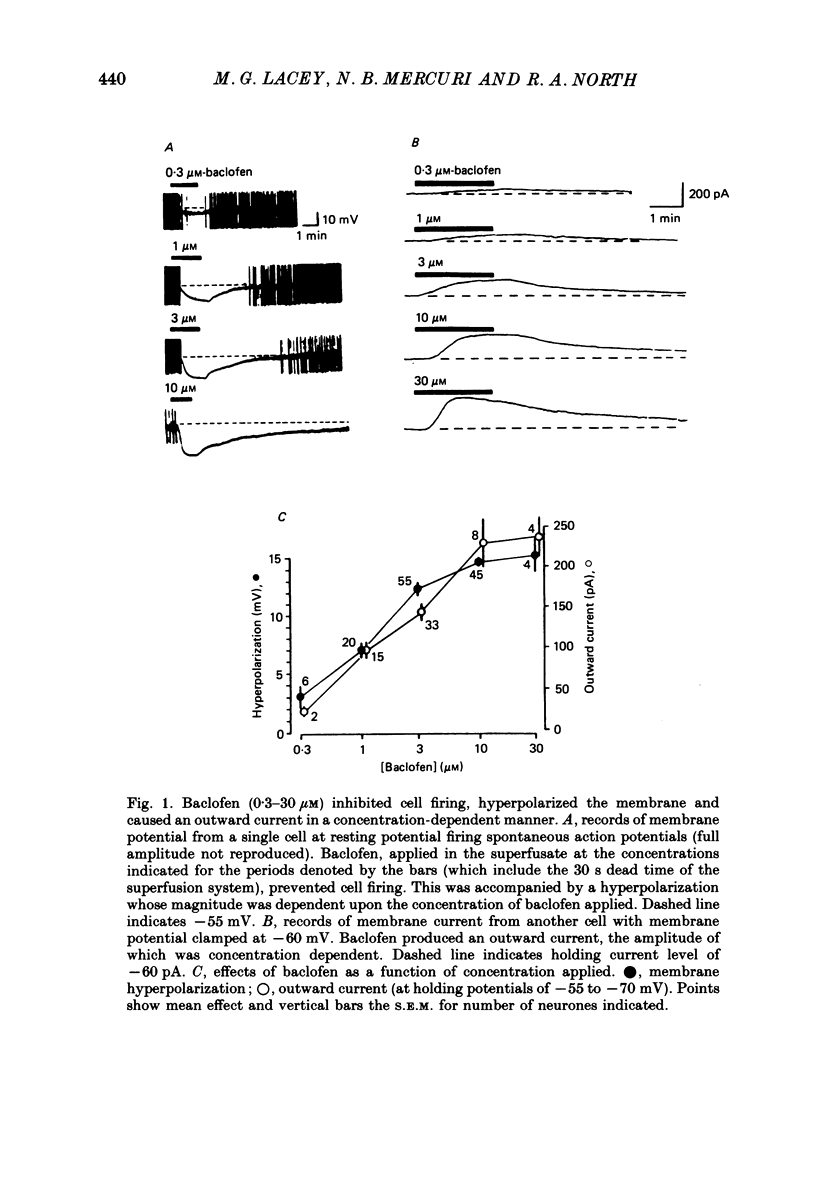
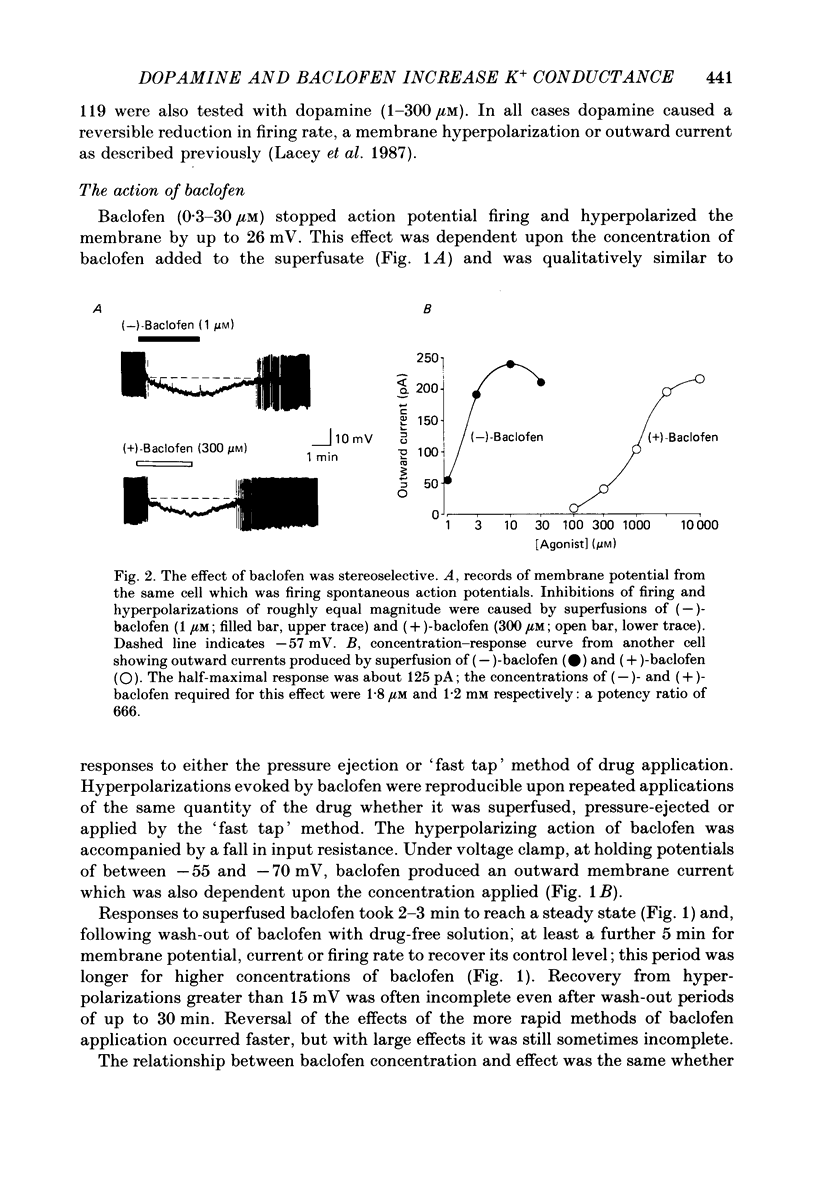
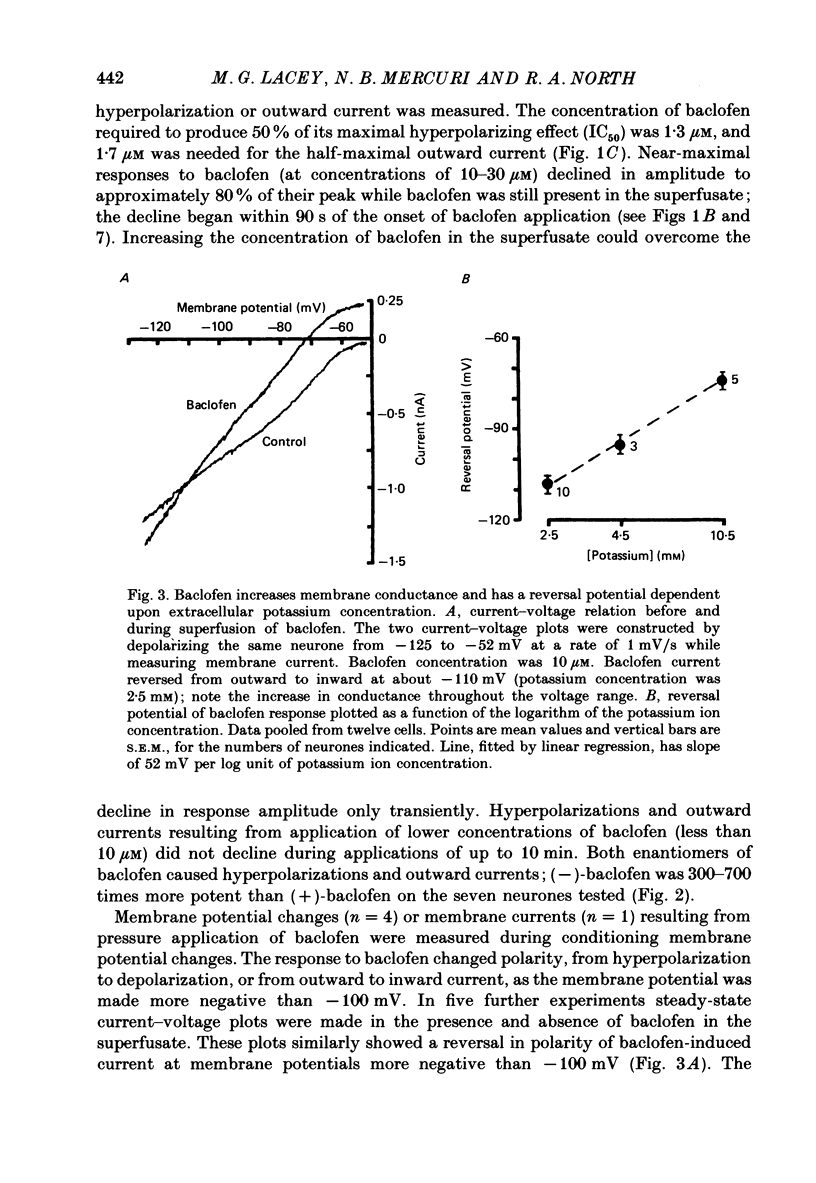
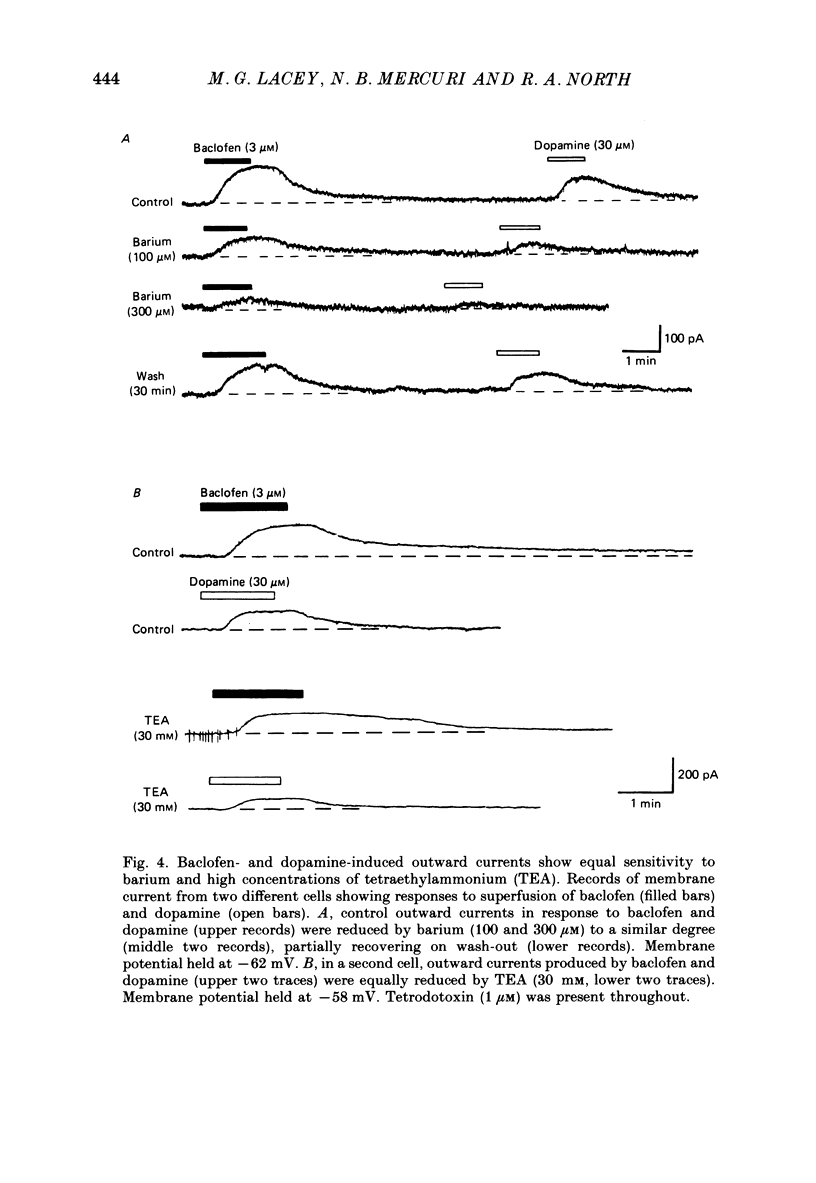
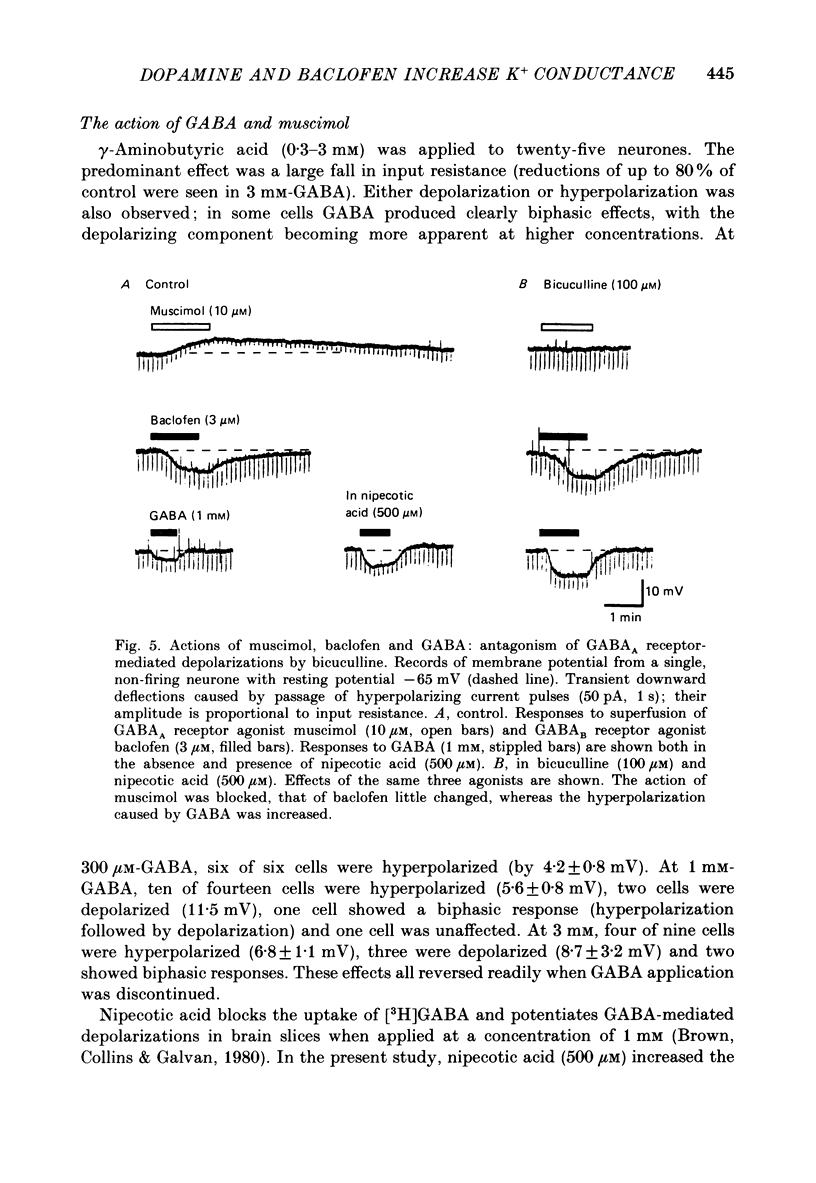
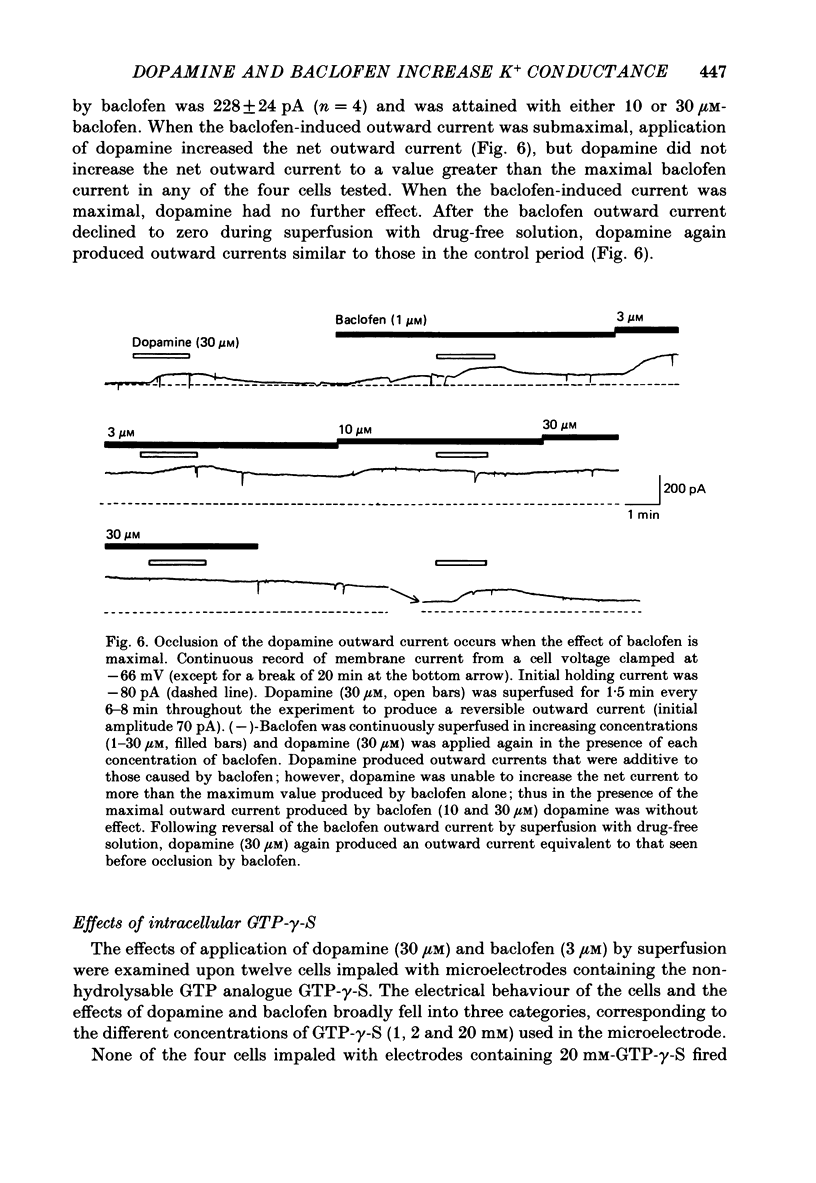
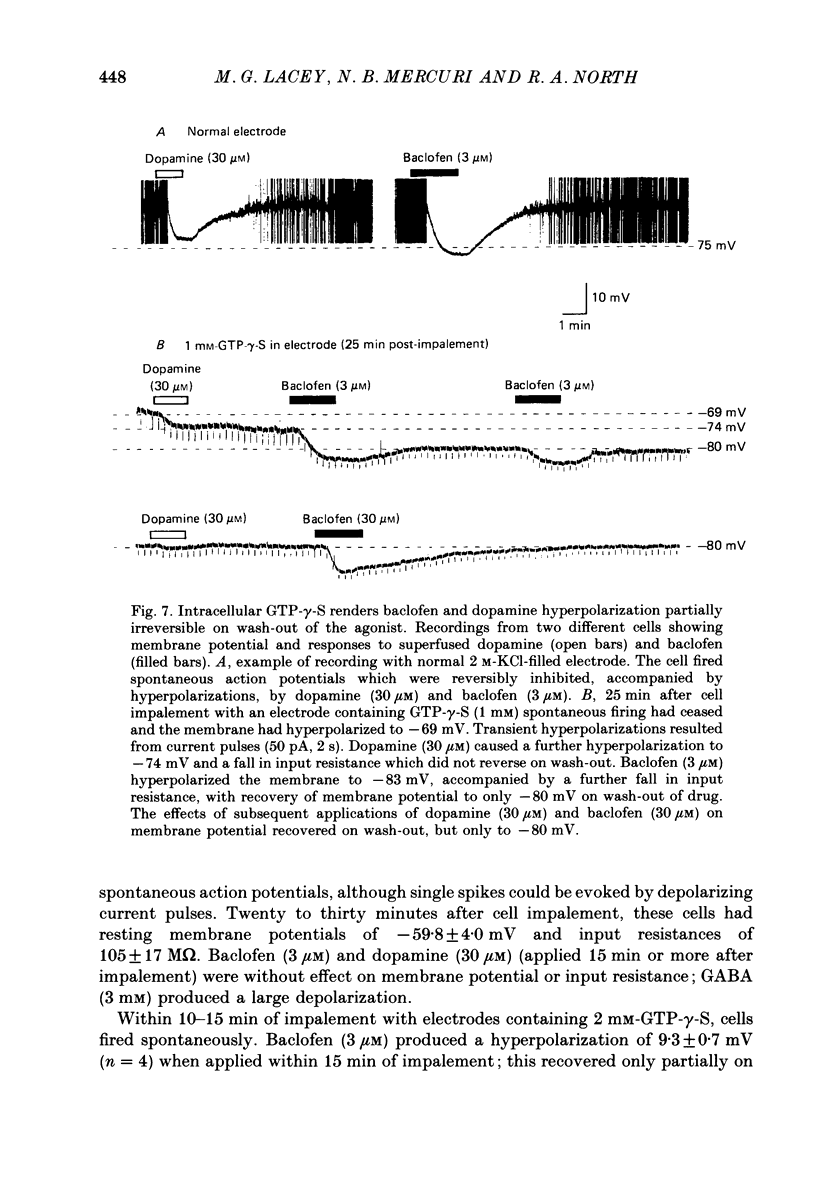
1. Intracellular recordings were made from 193 substantia nigra zona compacta neurones in slices of rat mesencephalon. All cells were hyperpolarized by baclofen; this was accompanied by a fall in input resistance. Cells voltage clamped at -60 mV showed an outward current associated with a conductance increase in response to baclofen. The baclofen effects were concentration dependent (effective range 0.3-30 microM); the concentration producing half the maximal effect was 1.5 microM. (-)-Baclofen was 300-700 times more potent than (+)-baclofen. 2. The potential change or membrane current caused by baclofen reversed polarity at -108.8 +/- 1.1 mV (n = 10) when the potassium ion concentration was 2.5 mM, -96.0 +/- 2.8 mV (n = 3) in 4.5 mM-potassium and -76.6 +/- 1.7 mV (n = 5) in 10.5 mM-potassium. The relationship between reversal potential and potassium concentration conformed to the Nernst equation. 3. Dopamine was also applied to 119 of these neurones; all exhibited either a hyperpolarization or an outward current. 4. Baclofen and dopamine outward currents were reduced reversibly by barium (100-300 microM) and tetraethylammonium (10 mM). Superfusion for 5-10 min with solutions presumed to block calcium currents reduced, but did not abolish, responses to baclofen. The effect of baclofen persisted in tetrodotoxin (1 microM). 5. Superfusion of gamma-aminobutyric acid (GABA, 0.3-3 mM) caused either membrane depolarization or hyperpolarization, accompanied by a fall in input resistance. The depolarization was mimicked by muscimol (10 microM) and blocked by bicuculline methiodide (10-100 microM); the hyperpolarization was resistant to bicuculline. Nipecotic acid (500 microM) enhanced the effect of GABA, but was without effect upon the actions of muscimol and baclofen. 6. The effect of dopamine was enhanced by cocaine (10 microM) and antagonized by (-)-sulpiride (0.1-1 microM), whereas the actions of baclofen were unaffected by cocaine or (-)-sulpiride. The maximum outward current produced by dopamine was approximately half that produced by baclofen. 7. Outward currents produced by dopamine were reversibly occluded by maximal outward currents caused by baclofen. 8. Baclofen and dopamine hyperpolarizations were unaffected by intracerebroventricular injection of animals with pertussis toxin. 9. Cells impaled with electrodes containing guanosine 5'-O-(3-thiotriphosphate) (1 mM) were hyperpolarized by both baclofen and dopamine, but the membrane potential did not fully return to its original level when agonist application was discontinued. 10. It is concluded that activation of both dopamine D2 and GABAB receptors may increase the same potassium conductance.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Wang Y. Y. Pertussis toxin blocks the outward currents evoked by opiate and alpha 2-agonists in locus coeruleus neurons. Brain Res. 1986 Apr 23;371(2):390–394. doi: 10.1016/0006-8993(86)90382-3. [DOI] [PubMed] [Google Scholar]
- Andrade R., Malenka R. C., Nicoll R. A. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986 Dec 5;234(4781):1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Axelrod J. Noradrenaline: fate and control of its biosynthesis. Science. 1971 Aug 13;173(3997):598–606. doi: 10.1126/science.173.3997.598. [DOI] [PubMed] [Google Scholar]
- Blaxter T. J., Carlen P. L., Davies M. F., Kujtan P. W. gamma-Aminobutyric acid hyperpolarizes rat hippocampal pyramidal cells through a calcium-dependent potassium conductance. J Physiol. 1986 Apr;373:181–194. doi: 10.1113/jphysiol.1986.sp016041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L. Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. Br J Pharmacol. 1983 Jan;78(1):191–206. doi: 10.1111/j.1476-5381.1983.tb09380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Collins G. G., Galvan M. Influence of cellular transport on the interaction of amino acids with gamma-aminobutyric acid (GABA)-receptors in the isolated olfactory cortex of the guinea-pig. Br J Pharmacol. 1980 Feb;68(2):251–262. doi: 10.1111/j.1476-5381.1980.tb10414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSSON A., LINDQVIST M. EFFECT OF CHLORPROMAZINE OR HALOPERIDOL ON FORMATION OF 3METHOXYTYRAMINE AND NORMETANEPHRINE IN MOUSE BRAIN. Acta Pharmacol Toxicol (Copenh) 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- Dray A. The striatum and substantia nigra: a commentary on their relationships. Neuroscience. 1979;4(10):1407–1439. doi: 10.1016/0306-4522(79)90048-4. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Sutor B., Zieglgänsberger W. Baclofen reduces post-synaptic potentials of rat cortical neurones by an action other than its hyperpolarizing action. J Physiol. 1987 Mar;384:539–569. doi: 10.1113/jphysiol.1987.sp016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis R. B., Aghajanian G. K. Pertussis toxin blocks autoreceptor-mediated inhibition of dopaminergic neurons in rat substantia nigra. Brain Res. 1987 May 12;411(1):139–143. doi: 10.1016/0006-8993(87)90690-1. [DOI] [PubMed] [Google Scholar]
- Inoue M., Matsuo T., Ogata N. Baclofen activates voltage-dependent and 4-aminopyridine sensitive K+ conductance in guinea-pig hippocampal pyramidal cells maintained in vitro. Br J Pharmacol. 1985 Apr;84(4):833–841. doi: 10.1111/j.1476-5381.1985.tb17377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol. 1987 Nov;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara S., North R. A., Surprenant A. Somatostatin increases an inwardly rectifying potassium conductance in guinea-pig submucous plexus neurones. J Physiol. 1987 Sep;390:335–355. doi: 10.1113/jphysiol.1987.sp016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Williams J. T. On the potassium conductance increased by opioids in rat locus coeruleus neurones. J Physiol. 1985 Jul;364:265–280. doi: 10.1113/jphysiol.1985.sp015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Williams J. T., Surprenant A., Christie M. J. Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5487–5491. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olpe H. R., Koella W. P., Wolf P., Haas H. L. The action of baclofen on neurons of the substantia nigra and of the ventral tegmental area. Brain Res. 1977 Oct 14;134(3):577–580. doi: 10.1016/0006-8993(77)90834-4. [DOI] [PubMed] [Google Scholar]
- Osmanović S. S., Shefner S. A. Baclofen increases the potassium conductance of rat locus coeruleus neurons recorded in brain slices. Brain Res. 1988 Jan 12;438(1-2):124–136. doi: 10.1016/0006-8993(88)91331-5. [DOI] [PubMed] [Google Scholar]
- Pinnock R. D. Hyperpolarizing action of baclofen on neurons in the rat substantia nigra slice. Brain Res. 1984 Nov 26;322(2):337–340. doi: 10.1016/0006-8993(84)90129-x. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Sato M. A single GTP-binding protein regulates K+-channels coupled with dopamine, histamine and acetylcholine receptors. Nature. 1987 Jan 15;325(6101):259–262. doi: 10.1038/325259a0. [DOI] [PubMed] [Google Scholar]
- Stevens D. R., Gallagher J. P., Shinnick-Gallagher P. Further studies on the action of baclofen on neurons of the dorsolateral septal nucleus of the rat, in vitro. Brain Res. 1985 Dec 9;358(1-2):360–363. doi: 10.1016/0006-8993(85)90984-9. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N., Smith A. D., Powell J. F. GABA axons in synaptic contact with dopamine neurons in the substantia nigra: double immunocytochemistry with biotin-peroxidase and protein A-colloidal gold. Brain Res. 1985 Nov 25;348(1):146–154. doi: 10.1016/0006-8993(85)90370-1. [DOI] [PubMed] [Google Scholar]


