Abstract
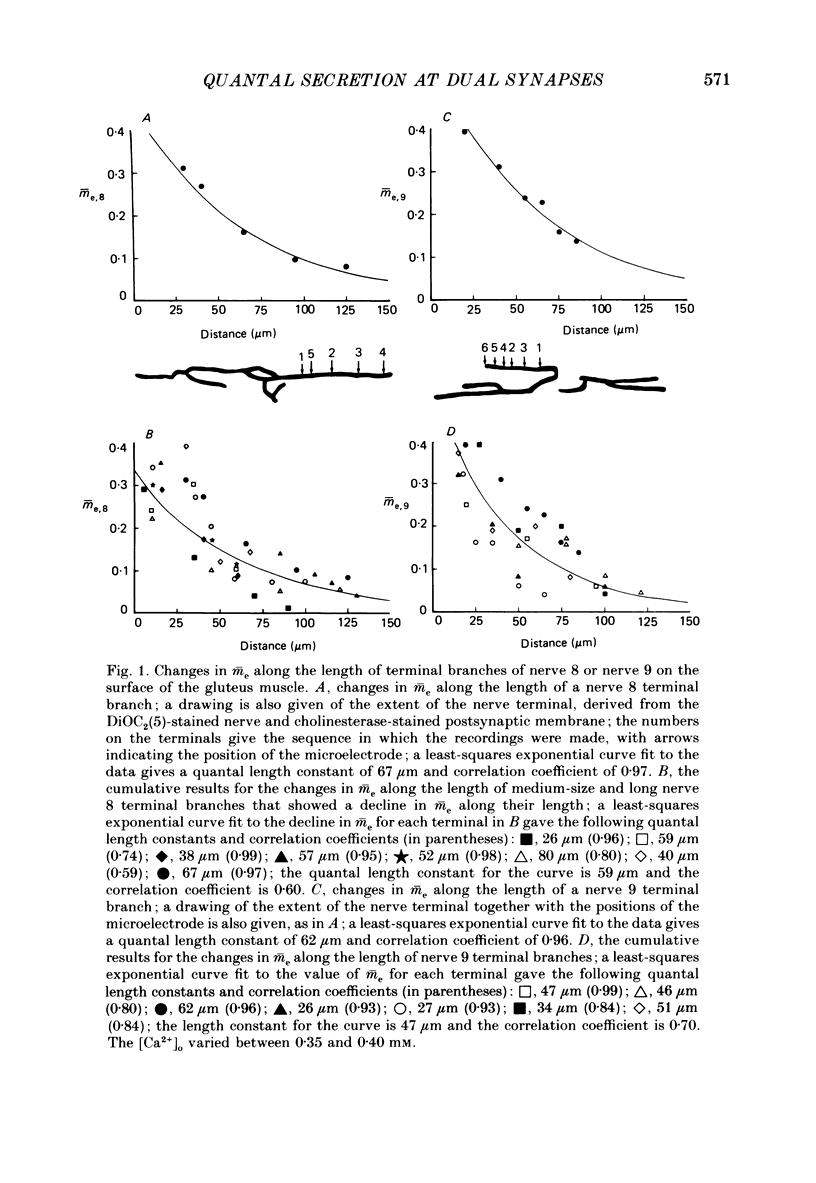
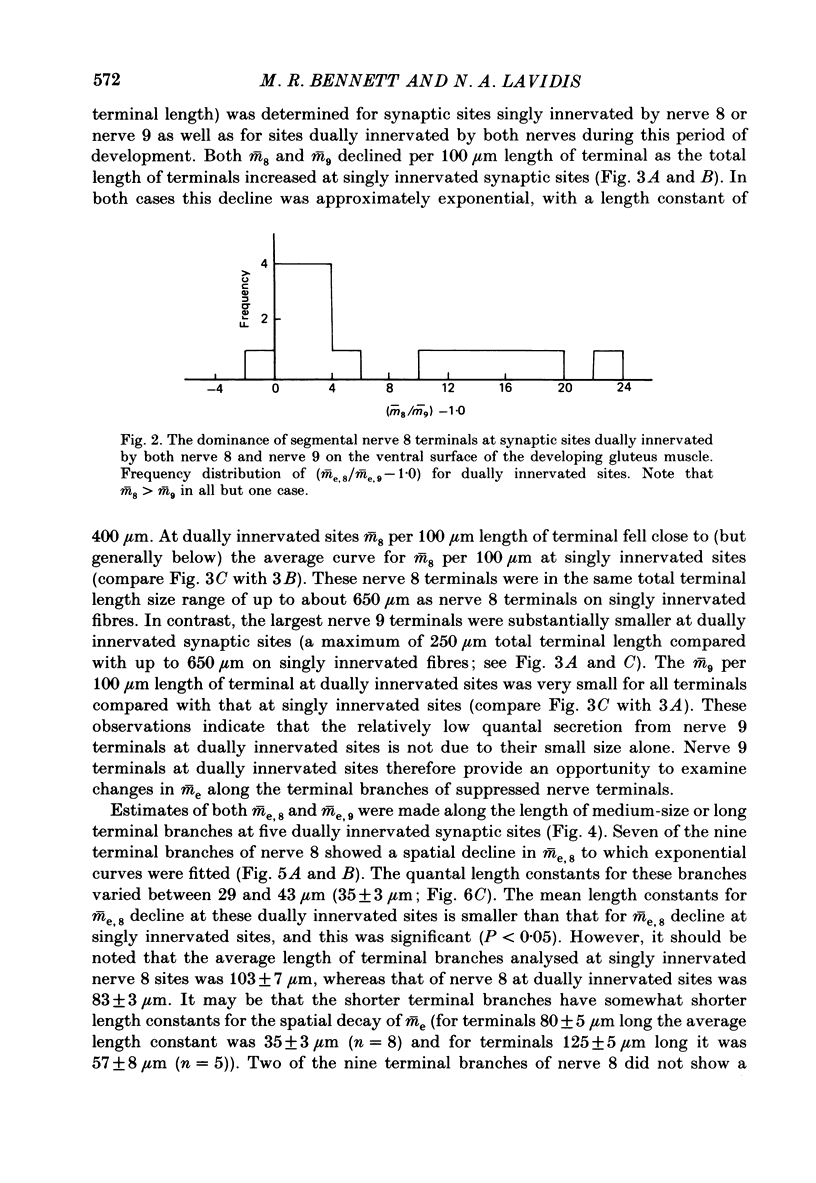
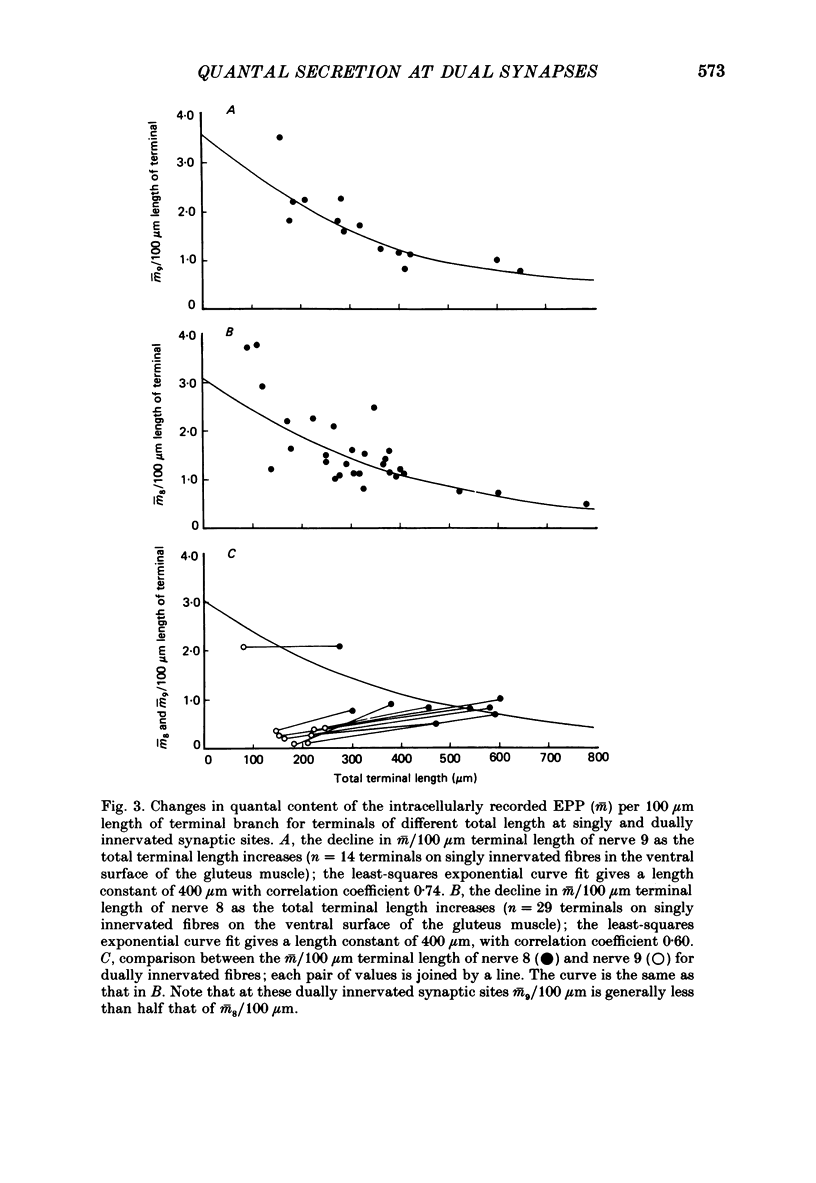
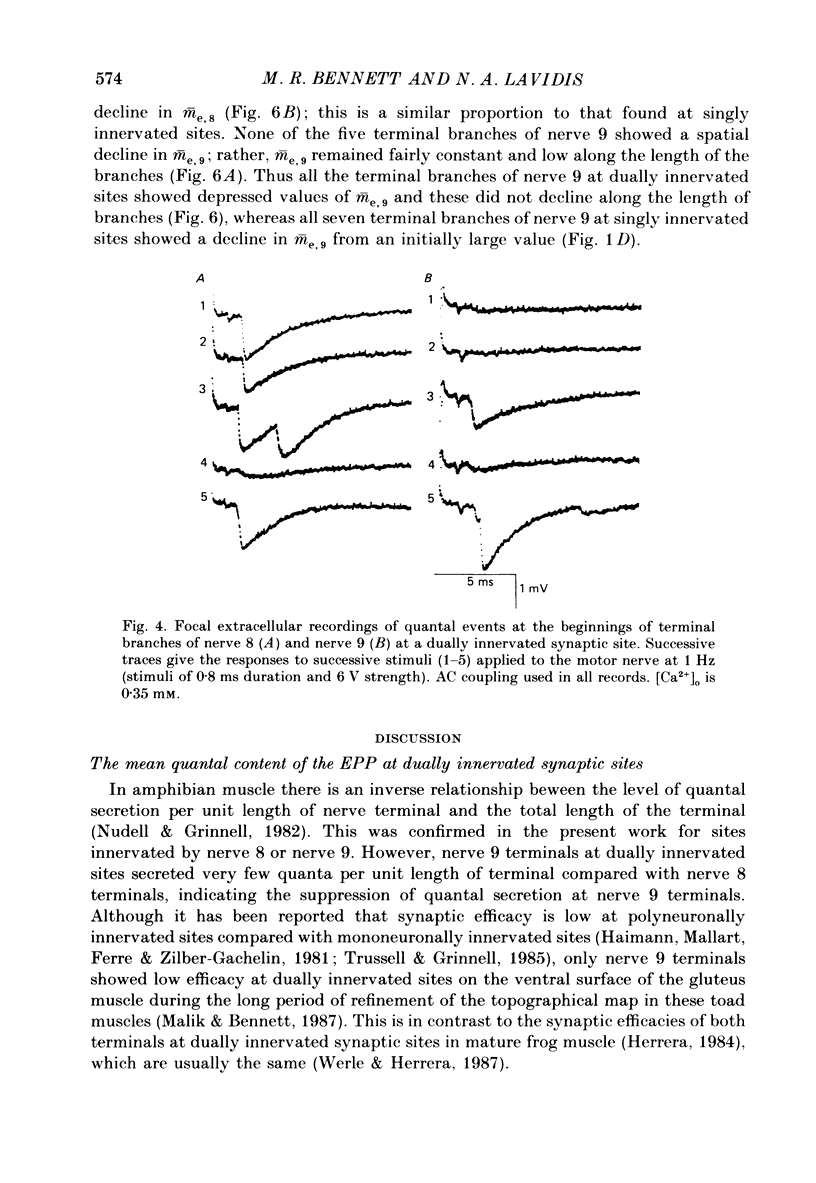
1. The number of quanta secreted from selected sites along terminal branches at suppressed synapses in the developing toad (Bufo marinus) gluteus muscle has been determined. The topographical projection from segmental nerves 8 and 9 to the ventral surface of this muscle matures slowly as toads develop in size from 12 to 40 g. Terminal branches of nerves 8 and 9 were visualized by prior staining with the fluorescent dye, 3-3-diethyloxardicarbocyanine iodide (DiOC2(5]. 2. The evoked quantal release recorded with an extracellular electrode (m(e) at different positions along the length of terminal branches at synaptic sites innervated either by nerve 8 (me,8) or nerve 9 (me,9) was determined in an external Ca2+ concentration, [Ca2+]o, of 0.35-0.45 mM. For over 90% of branches longer than 80 microns, me declined along exponential curves from a relatively large value at the proximal end of branches for both nerve 8 and nerve 9 terminals; the exponent for these exponential curves gave quantal length constants that varied from 26 to 80 microns (48 +/- 4 microns, mean +/- S.E.M.) depending on the length of the branch. 3. The evoked quantal release recorded with an intracellular electrode (m) at synaptic sites dually innervated by nerve 8 and nerve 9 was nearly always (greater than 90%) greater for nerve 8 terminals than for nerve 9 terminals. At singly innervated sites the value of m per 100 microns length of terminal declined approximately exponentially with an increase in total terminal length (length constant 400 microns). However, at dually innervated sites the value of m per 100 microns length of nerve 9 terminal was very low at all total terminal lengths compared with singly innervated sites; this indicates that nerve 9 terminals were suppressed at dually innervated sites. 4. At five dually innervated sites, seven out of nine terminal branches of nerve 8 showed an exponential decline in me,8 along their length, from a relatively large value near the proximal end of the branches (length constant 35 +/- 3 microns, mean +/- S.E.M.). In contrast, all the terminal branches of nerve 9 greater than 80 microns showed a uniformly low value of me,9 along their length. 5. It is suggested that the suppression of nerve 9 terminals at dually innervated sites is primarily due to a decrease in the probability of secretion of normally highly secreting release sites at the proximal end of terminal branches.
Full text
PDF



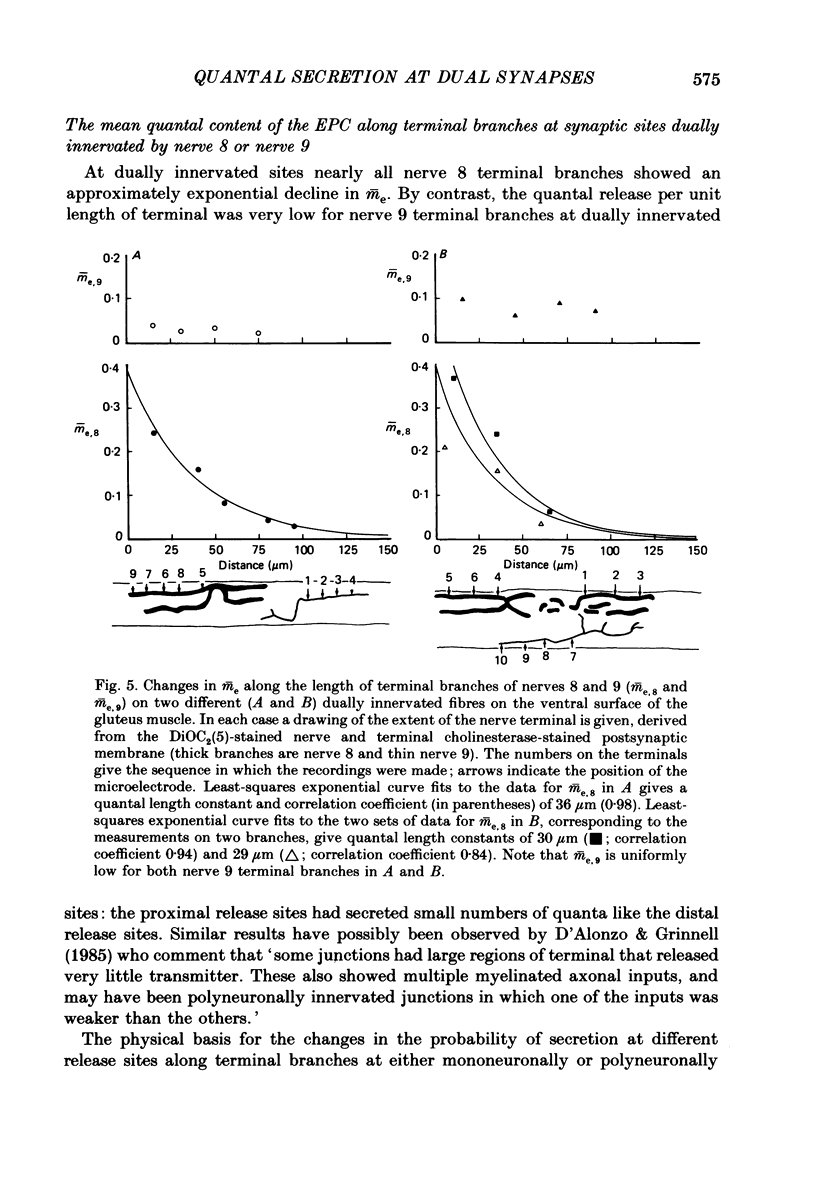
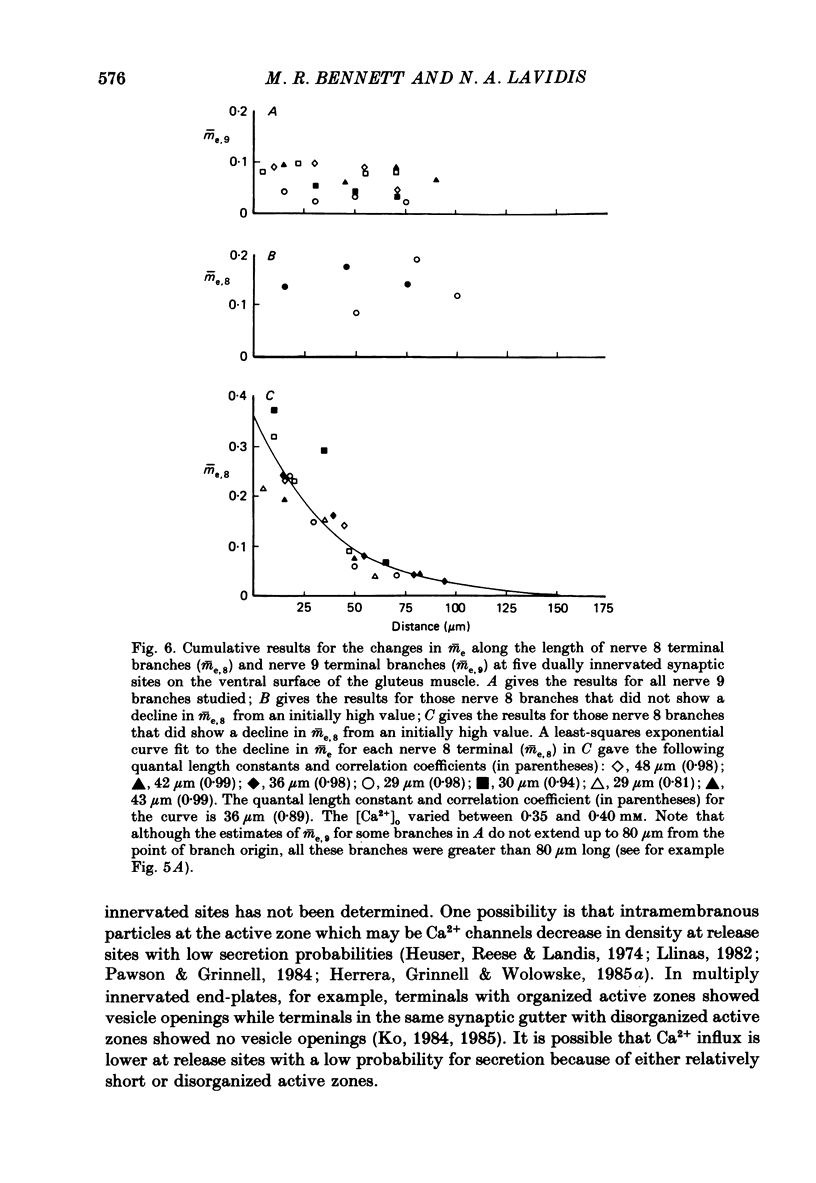



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett E. F., Stevens C. F. Quantal independence and uniformity of presynaptic release kinetics at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):665–689. doi: 10.1113/jphysiol.1972.sp010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R. Development of neuromuscular synapses. Physiol Rev. 1983 Jul;63(3):915–1048. doi: 10.1152/physrev.1983.63.3.915. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Jones P., Lavidis N. A. The probability of quantal secretion along visualized terminal branches at amphibian (Bufo marinus) neuromuscular synapses. J Physiol. 1986 Oct;379:257–274. doi: 10.1113/jphysiol.1986.sp016252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Lavidis N. A., Armson F. M. Changes in the dimensions of release sites along terminal branches at amphibian neuromuscular synapses. J Neurocytol. 1987 Apr;16(2):221–237. doi: 10.1007/BF01795306. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Lavidis N. A. The effect of calcium ions on the secretion of quanta evoked by an impulse at nerve terminal release sites. J Gen Physiol. 1979 Oct;74(4):429–456. doi: 10.1085/jgp.74.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in amphibian striated muscle during development. J Physiol. 1975 Oct;252(1):203–239. doi: 10.1113/jphysiol.1975.sp011141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M., Lavidis N. Topographical projections of segmental nerves to the frog glutaeus muscle during loss of polyneuronal innervation. J Physiol. 1986 Jun;375:303–325. doi: 10.1113/jphysiol.1986.sp016118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieser A., Wernig A., Zucker H. Different quantal responses within single frog neuromuscular junctions. J Physiol. 1984 May;350:401–412. doi: 10.1113/jphysiol.1984.sp015208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couteaux R., Pécot-Dechavassine M. Vésicules synaptiques et poches au niveau des "zones actives" de la jonction neuromusculaire. C R Acad Sci Hebd Seances Acad Sci D. 1970 Dec 21;271(25):2346–2349. [PubMed] [Google Scholar]
- D'Alonzo A. J., Grinnell A. D. Profiles of evoked release along the length of frog motor nerve terminals. J Physiol. 1985 Feb;359:235–258. doi: 10.1113/jphysiol.1985.sp015583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Localization of active spots within the neuromuscular junction of the frog. J Physiol. 1956 Jun 28;132(3):630–649. doi: 10.1113/jphysiol.1956.sp005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey D. F., Bennett M. R. Variation in the size of synaptic contacts along developing and mature motor terminal branches. Brain Res. 1982 Sep;281(1):11–22. doi: 10.1016/0165-3806(82)90108-0. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K., Akert K., Sandri C., Moor H. Ultrastructure of the "active zone" in the frog neuromuscular junction. Brain Res. 1973 Nov 23;62(2):373–380. doi: 10.1016/0006-8993(73)90699-9. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimann C., Mallart A., Ferré J. T., Zilber-Gachelin N. F. Interaction between motor axons from two different nerves reinnervating the pectoral muscle of Xenopus laevis. J Physiol. 1981 Jan;310:257–272. doi: 10.1113/jphysiol.1981.sp013547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A. A., Grinnell A. D., Wolowske B. Ultrastructural correlates of experimentally altered transmitter release efficacy in frog motor nerve terminals. Neuroscience. 1985 Nov;16(3):491–500. doi: 10.1016/0306-4522(85)90187-3. [DOI] [PubMed] [Google Scholar]
- Herrera A. A., Grinnell A. D., Wolowske B. Ultrastructural correlates of naturally occurring differences in transmitter release efficacy in frog motor nerve terminals. J Neurocytol. 1985 Apr;14(2):193–202. doi: 10.1007/BF01258447. [DOI] [PubMed] [Google Scholar]
- Herrera A. A. Polyneuronal innervation and quantal transmitter release in formamide-treated frog sartorius muscles. J Physiol. 1984 Oct;355:267–280. doi: 10.1113/jphysiol.1984.sp015418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Landis D. M. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J Neurocytol. 1974 Mar;3(1):109–131. doi: 10.1007/BF01111936. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J. THE LOCALIZATION OF CHOLINESTERASE ACTIVITY IN RAT CARDIAC MUSCLE BY ELECTRON MICROSCOPY. J Cell Biol. 1964 Nov;23:217–232. doi: 10.1083/jcb.23.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Ko C. P. Formation of the active zone at developing neuromuscular junctions in larval and adult bullfrogs. J Neurocytol. 1985 Jun;14(3):487–512. doi: 10.1007/BF01217757. [DOI] [PubMed] [Google Scholar]
- Ko C. P. Regeneration of the active zone at the frog neuromuscular junction. J Cell Biol. 1984 May;98(5):1685–1695. doi: 10.1083/jcb.98.5.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinsky M. S., Morrison-Graham K. Structure of developing frog neuromuscular junctions. J Neurocytol. 1980 Jun;9(3):321–342. doi: 10.1007/BF01181540. [DOI] [PubMed] [Google Scholar]
- Llinás R. R. Calcium in synaptic transmission. Sci Am. 1982 Oct;247(4):56–65. doi: 10.1038/scientificamerican1082-56. [DOI] [PubMed] [Google Scholar]
- Magrassi L., Purves D., Lichtman J. W. Fluorescent probes that stain living nerve terminals. J Neurosci. 1987 Apr;7(4):1207–1214. doi: 10.1523/JNEUROSCI.07-04-01207.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R., Bennett M. R. Loss of polyneuronal innervation and establishment of a topographical map in the glutaeus muscle of Bufo marinus during generation of secondary muscle cells. Brain Res. 1987 Aug;431(2):173–189. doi: 10.1016/0165-3806(87)90207-0. [DOI] [PubMed] [Google Scholar]
- Miller T. M., Heuser J. E. Endocytosis of synaptic vesicle membrane at the frog neuromuscular junction. J Cell Biol. 1984 Feb;98(2):685–698. doi: 10.1083/jcb.98.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison-Graham K. An anatomical and electrophysiological study of synapse elimination at the developing frog neuromuscular junction. Dev Biol. 1983 Oct;99(2):298–311. doi: 10.1016/0012-1606(83)90279-8. [DOI] [PubMed] [Google Scholar]
- Nudell B. M., Grinnell A. D. Inverse relationship between transmitter release and terminal length in synapses on frog muscle fibers of uniform input resistance. J Neurosci. 1982 Feb;2(2):216–224. doi: 10.1523/JNEUROSCI.02-02-00216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson P. A., Grinnell A. D. Posttetanic potentiation in strong and weak neuromuscular junctions: physiological differences caused by a differential Ca2+-influx. Brain Res. 1984 Dec 10;323(2):311–315. doi: 10.1016/0006-8993(84)90304-4. [DOI] [PubMed] [Google Scholar]
- Robitaille R., Tremblay J. P. Non-uniform release at the frog neuromuscular junction: evidence of morphological and physiological plasticity. Brain Res. 1987 Mar;434(1):95–116. doi: 10.1016/0165-0173(87)90019-1. [DOI] [PubMed] [Google Scholar]
- Sherrington C. S. Notes on the Arrangement of some Motor Fibres in the Lumbo-Sacral Plexus. J Physiol. 1892 Oct;13(6):621–772.17. doi: 10.1113/jphysiol.1892.sp000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell L. O., Grinnell A. D. The regulation of synaptic strength within motor units of the frog cutaneous pectoris muscle. J Neurosci. 1985 Jan;5(1):243–254. doi: 10.1523/JNEUROSCI.05-01-00243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werle M. J., Herrera A. A. Synaptic competition and the persistence of polyneuronal innervation at frog neuromuscular junctions. J Neurobiol. 1987 Jul;18(4):375–389. doi: 10.1002/neu.480180405. [DOI] [PubMed] [Google Scholar]
- Yoshikami D., Okun L. M. Staining of living presynaptic nerve terminals with selective fluorescent dyes. Nature. 1984 Jul 5;310(5972):53–56. doi: 10.1038/310053a0. [DOI] [PubMed] [Google Scholar]
- Zefirov A. L., Stolov E. L. Model' sekretsii mediatora v nervno-myshechnom sinapse, osnovannaia na prostranstvennoi neodonorodnosti veroiatnosti osvobozhdeniia kvanta atsetilkholina. Neirofiziologiia. 1982;14(3):233–240. [PubMed] [Google Scholar]


