Abstract
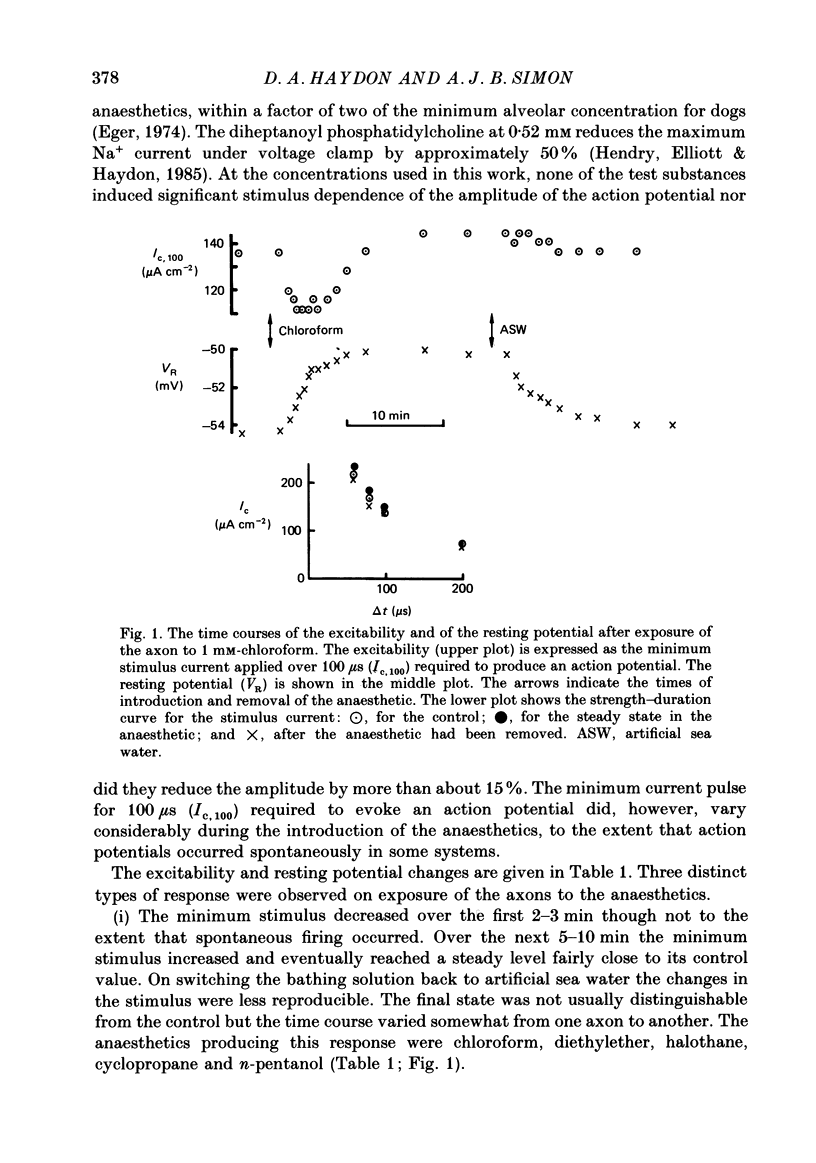
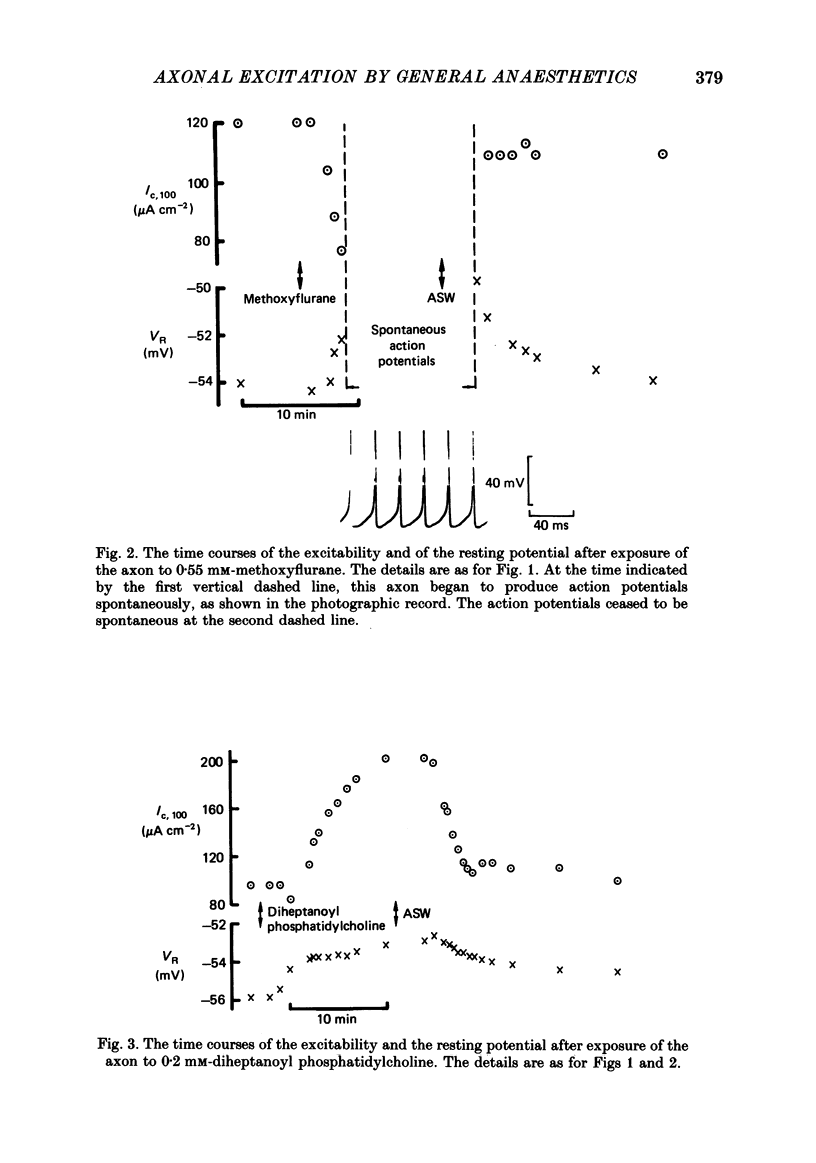
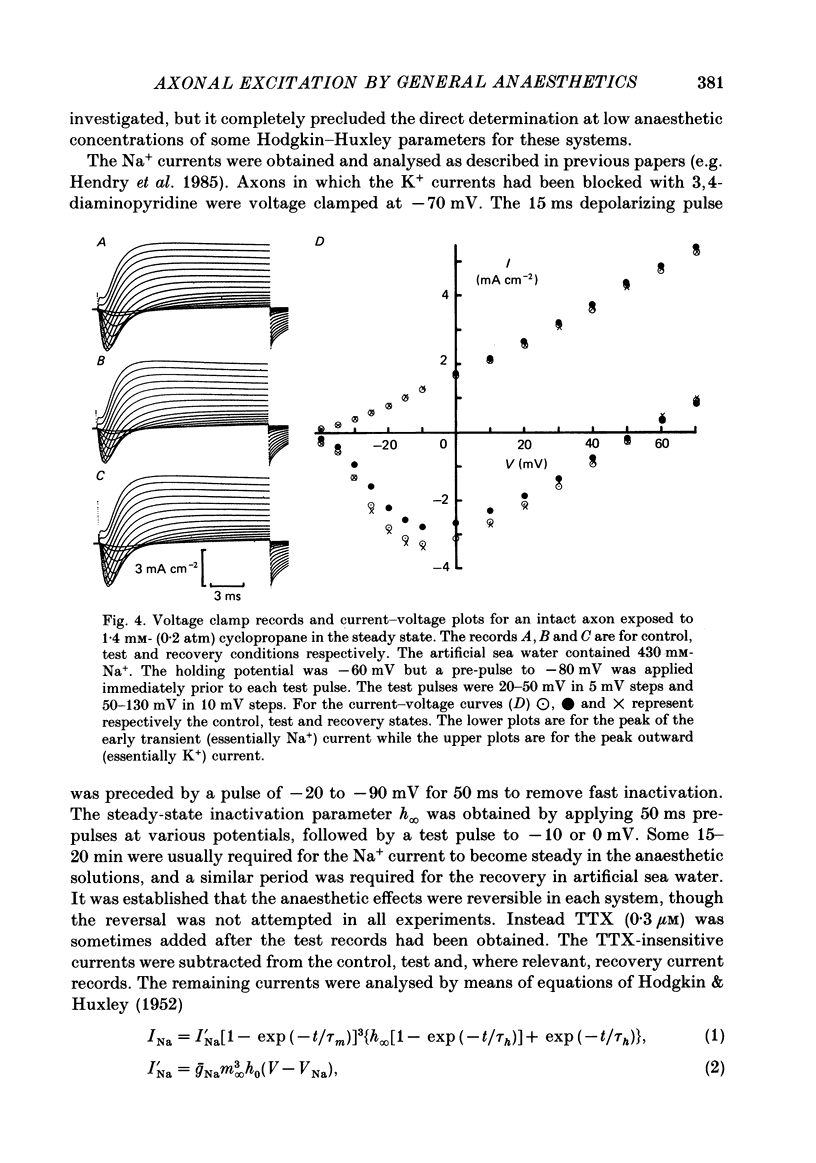
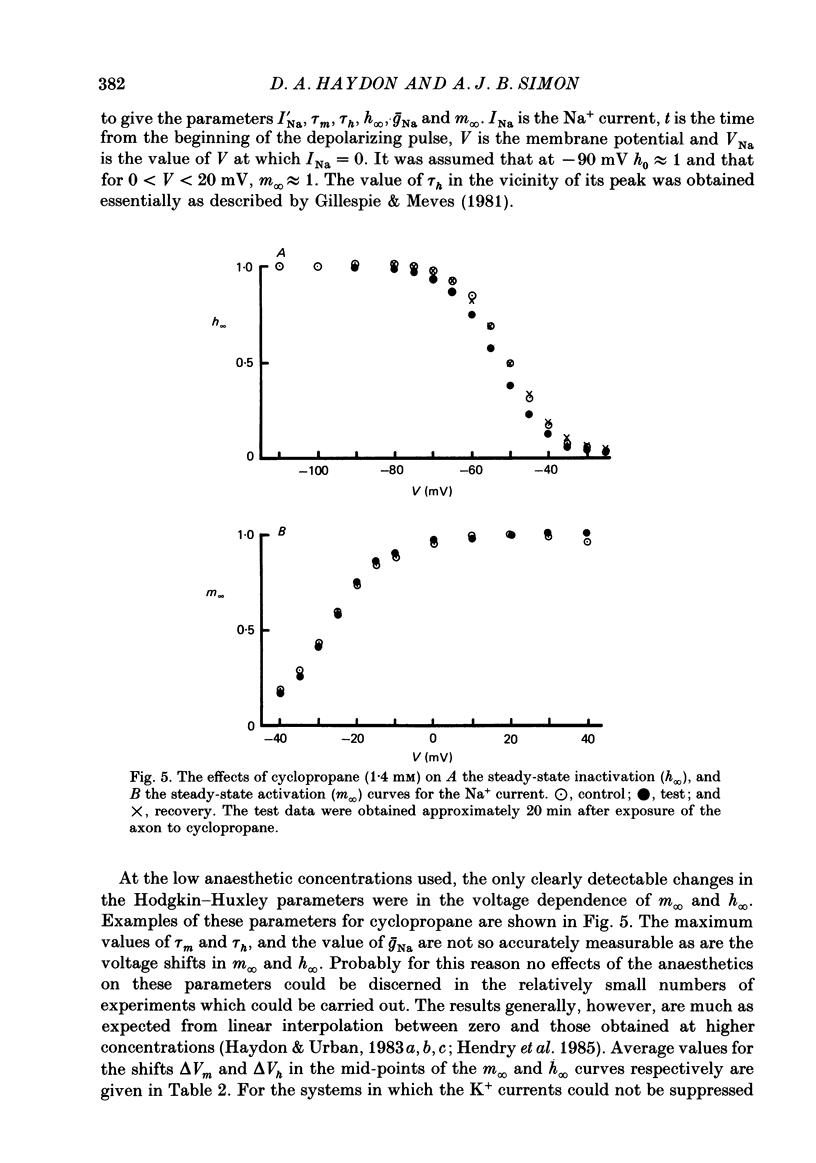
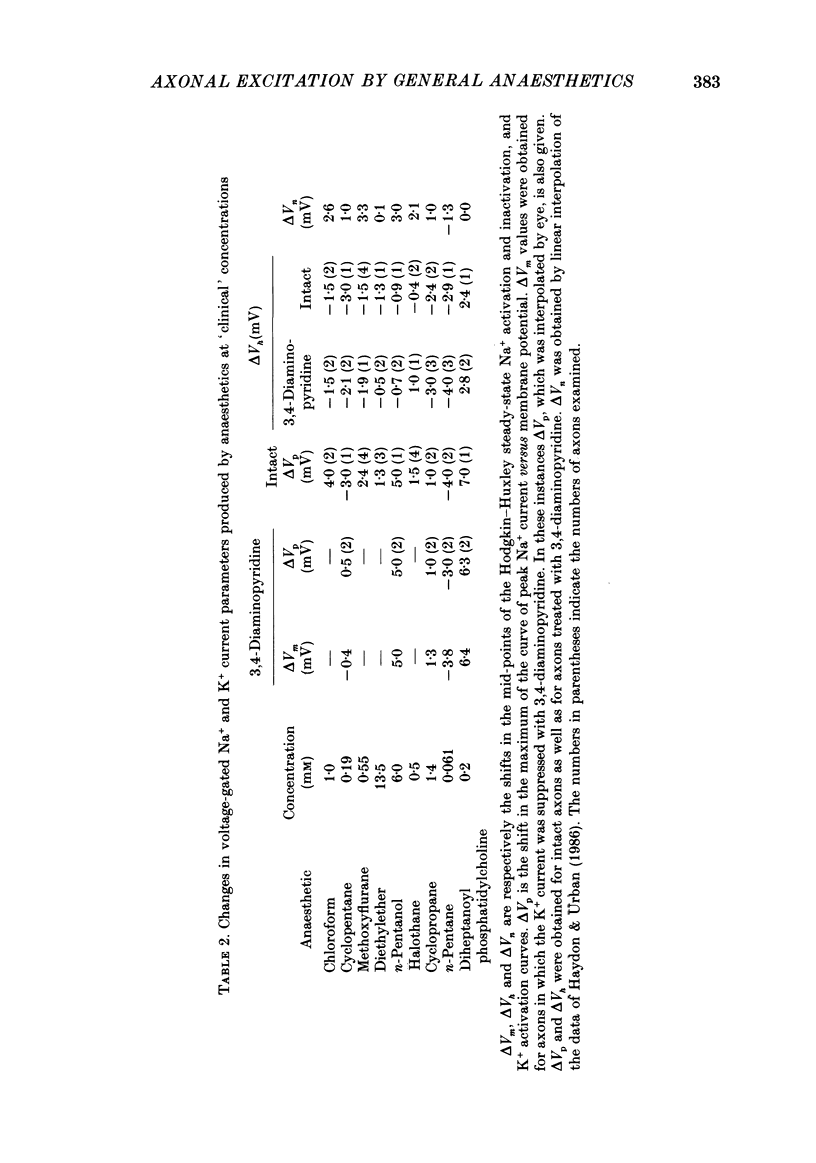
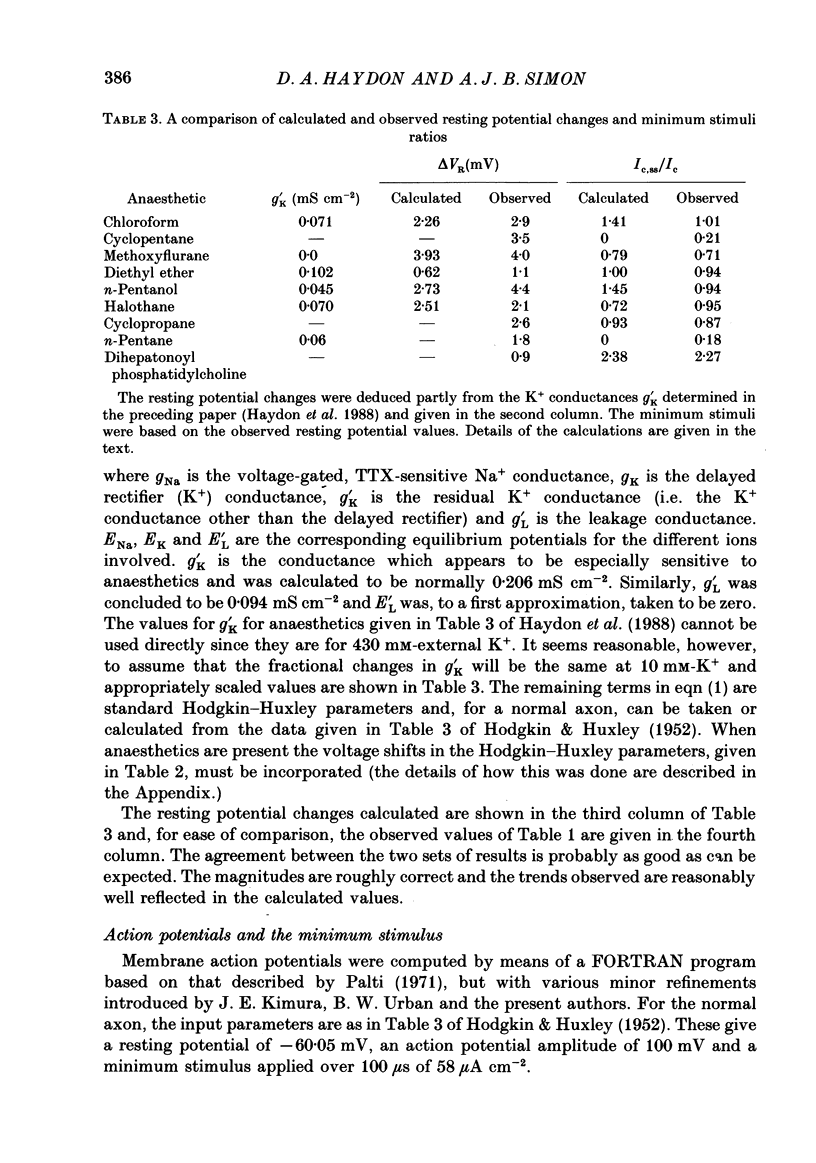
1. The effects of 'clinical' concentrations of some general anaesthetics on the minimum stimulus required to produce an action potential in the squid giant axon have been examined as a function of time from exposure to the anaesthetic. The resting potential in these experiments was also monitored. 2. The minimum stimulus varied with time in different ways for different anaesthetics. For chloroform, diethyl ether, n-pentanol, halothane and cyclopropane the stimulus initially declined, reached a minimum after about 3 min and then recovered to near-normal values at 10-15 min. For n-pentane, cyclopentane and, to a lesser extent methoxyflurane, the stimulus often declined to such low values that the axon exhibited spontaneous action potentials which persisted until the anaesthetic was removed. For one substance, the experimental local anaesthetic diheptanoyl phosphatidylcholine, the stimulus increased considerably over the 10-15 min required to reach the steady state. In all instances the axons reverted to normal behaviour after removal of the anaesthetic although the time course by which they did so was more variable than for the initial exposure. 3. For all anaesthetics the resting potential changed in the positive direction monotonically by ca. 1-5 mV and reached a steady state in approximately 3 min. On removal of the anaesthetic the resting potential returned to normal, also monotonically. 4. The voltage-gated Na+ and K+ currents were significantly affected even at the low anaesthetic concentrations used. Estimates of the changes in the Hodgkin-Huxley parameters were obtained partly by direct experiment and partly from results previously obtained for higher anaesthetic concentrations. 5. The time dependencies of the minimum stimuli have been accounted for semi-quantitatively in terms of the resting potential changes and the voltage shifts in the Na+ current steady-state activation, and the time dependencies respectively of these two parameters. 6. Quantitative calculations of the resting potential changes for comparison with experiment have been made based on the changes in K+ conductance determined in the preceding paper (Haydon, Requena & Simon, 1988) and changes in the Hodgkin-Huxley parameters of the Na+ and delayed-rectifier K+ currents. 7. Calculations of the minimum stimulus in the steady state have been made from the experimental resting potential changes and from the anaesthetic-affected Hodgkin-Huxley parameters. Good agreement with the experimental stimuli was found, especially in the prediction of high and low values.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DIGIOVANNI A. J., DRIPPS R. D. Abnormal motor movements during divinyl ether anesthesia. Anesthesiology. 1956 Mar;17(2):353–357. doi: 10.1097/00000542-195603000-00015. [DOI] [PubMed] [Google Scholar]
- Gillespie J. I., Meves H. The effect of external potassium on the removal of sodium inactivation in squid giant axons. J Physiol. 1981 Jun;315:493–514. doi: 10.1113/jphysiol.1981.sp013760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Hendry B. M., Levinson S. R., Requena J. Anaesthesia by the n-alkanes. A comparative study of nerve impulse blockage and the properties of black lipid bilayer membranes. Biochim Biophys Acta. 1977 Oct 3;470(1):17–34. doi: 10.1016/0005-2736(77)90058-x. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Kimura J. E. Some effects of n-pentane on the sodium and potassium currents of the squid giant axon. J Physiol. 1981 Mar;312:57–70. doi: 10.1113/jphysiol.1981.sp013615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Requena J., Simon A. J. The potassium conductance of the resting squid axon and its blockage by clinical concentrations of general anaesthetics. J Physiol. 1988 Aug;402:363–374. doi: 10.1113/jphysiol.1988.sp017209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Requena J., Urban B. W. Some effects of aliphatic hydrocarbons on the electrical capacity and ionic currents of the squid giant axon membrane. J Physiol. 1980 Dec;309:229–245. doi: 10.1113/jphysiol.1980.sp013506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The action of alcohols and other non-ionic surface active substances on the sodium current of the squid giant axon. J Physiol. 1983 Aug;341:411–427. doi: 10.1113/jphysiol.1983.sp014813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The action of hydrocarbons and carbon tetrachloride on the sodium current of the squid giant axon. J Physiol. 1983 May;338:435–450. doi: 10.1113/jphysiol.1983.sp014682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The actions of some general anaesthetics on the potassium current of the squid giant axon. J Physiol. 1986 Apr;373:311–327. doi: 10.1113/jphysiol.1986.sp016049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The effects of some inhalation anaesthetics on the sodium current of the squid giant axon. J Physiol. 1983 Aug;341:429–439. doi: 10.1113/jphysiol.1983.sp014814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry B. M., Elliott J. R., Haydon D. A. Further evidence that membrane thickness influences voltage-gated sodium channels. Biophys J. 1985 Jun;47(6):841–845. doi: 10.1016/S0006-3495(85)83988-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendig J. J., Bunker J. P. Alterations in muscle resting potentials and electrolytes during halothane and cyclopropane anesthesia. Anesthesiology. 1972 Feb;36(2):128–131. doi: 10.1097/00000542-197202000-00008. [DOI] [PubMed] [Google Scholar]
- Meves H. Inactivation of the sodium permeability in squid giant nerve fibres. Prog Biophys Mol Biol. 1978;33(2):207–230. doi: 10.1016/0079-6107(79)90029-4. [DOI] [PubMed] [Google Scholar]
- PITTINGER C., MITCHELL C., ALEU F., PAGE W. Convulsive phenomena in hyperthermic dogs during anesthesia. Anesthesiology. 1961 Nov-Dec;22:893–896. doi: 10.1097/00000542-196111000-00004. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Sevcik C. Differences between the actions of thiopental and pentobarbital in squid giant axons. J Pharmacol Exp Ther. 1980 Sep;214(3):657–663. [PubMed] [Google Scholar]
- Urban B. W., Haydon D. A. The actions of halogenated ethers on the ionic currents of the squid giant axon. Proc R Soc Lond B Biol Sci. 1987 Jun 22;231(1262):13–26. doi: 10.1098/rspb.1987.0032. [DOI] [PubMed] [Google Scholar]


