Abstract
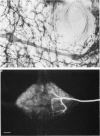
1. The membrane properties, morphology and physiological responses of peripherally located sensory neurones that innervate body wall muscle of the leech Hirudo medicinalis have been investigated using intracellular recording and dye injection techniques. 2. The peripheral neurones and their dendrites were visualized directly in whole mounts of the body wall by intracellular injection of horseradish peroxidase or Lucifer Yellow. They lie along the course of segmental nerves between the layers of longitudinal and oblique body wall muscle and within the sheath of the nerve. They have a distinctive morphology with two expanded, fan-shaped dendrites arranged in series separated by the cell body and a 300 micron long cylindrical process. Both dendrites are associated with longitudinal muscle of the ventral body wall but with separate bands of muscle fibres. The axons project into the ventral nerve cord and arborize within the ipsilateral half of the segmental ganglion. No processes extend across the mid-line of the ganglion or enter the connectives to neighbouring ganglia. 3. 'Resting' membrane potentials recorded from the peripheral cell body or from the axon as it entered the segmental ganglion ranged from -30 to -70 mV. The transmembrane potential recorded depended on the amount by which the body wall was stretched: the most hyperpolarized values were recorded from the most stretched preparations. Although the peripheral cell body can generate overshooting action potentials these are not actively propagated to the CNS. Rather, imposed voltage changes spread decrementally along the axon. Input resistances measured in the cell body ranged from 14 to 26 M omega. The space constant, estimated from the spread of hyperpolarizing current injected into the cell body, was 2.4 mm. 4. The response of the neurones to change in length of the longitudinal muscle recorded from the axon near its terminal arborization within the ventral nerve cord is a graded DC signal: the neurones thus relay information to CNS synapses in analogue form. Spiking activity recorded extracellularly in the anterior segmental nerve root in response to stretch of the body wall is due to activation of touch mechanosensory cells that innervate the skin. 5. Unlike stretch receptors innervating skeletal muscle in vertebrates or arthropods, the leech neurones respond to stretch of the body wall muscle with maintained hyperpolarizing potentials and to release of stretch with depolarization.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Nicholls J. G. Chemical and electrical synaptic connexions between cutaneous mechanoreceptor neurones in the central nervous system of the leech. J Physiol. 1969 Aug;203(3):591–609. doi: 10.1113/jphysiol.1969.sp008881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S. E. Morphology and distribution of touch cell terminals in the skin of the leech. J Physiol. 1981 Nov;320:219–228. doi: 10.1113/jphysiol.1981.sp013945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S. E., Nicholls J. G., Parnas I. Physiological responses, receptive fields and terminal arborizations of nociceptive cells in the leech. J Physiol. 1982 May;326:251–260. doi: 10.1113/jphysiol.1982.sp014189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blight A. R., Llinás R. The non-impulsive stretch-receptor complex of the crab: a study of depolarization--release coupling at a tonic sensorimotor synapse. Proc Clin Dial Transplant Forum. 1980 Jul 31;290(1039):219–276. doi: 10.1098/rstb.1980.0092. [DOI] [PubMed] [Google Scholar]
- Granzow B., Friesen W. O., Kristan W. B., Jr Physiological and morphological analysis of synaptic transmission between leech motor neurons. J Neurosci. 1985 Aug;5(8):2035–2050. doi: 10.1523/JNEUROSCI.05-08-02035.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J., Corey D. P. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2407–2411. doi: 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristan W. B., Jr, Weeks J. C. Neurons controlling the initiation, generation and modulation of leech swimming. Symp Soc Exp Biol. 1983;37:243–260. [PubMed] [Google Scholar]
- Laverack M. S. Mechanoreceptors, photoreceptors and rapid conduction pathways in the leech, Hirudo medicinalis. J Exp Biol. 1969 Feb;50(1):129–140. doi: 10.1242/jeb.50.1.129. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirolli M. The electrical properties of a crustacean sensory dendrite. J Exp Biol. 1979 Feb;78:1–27. doi: 10.1242/jeb.78.1.1. [DOI] [PubMed] [Google Scholar]
- Muller K. J., McMahan U. J. The shapes of sensory and motor neurones and the distribution of their synapses in ganglia of the leech: a study using intracellular injection of horseradish peroxidase. Proc R Soc Lond B Biol Sci. 1976 Nov 12;194(1117):481–499. doi: 10.1098/rspb.1976.0090. [DOI] [PubMed] [Google Scholar]
- Nicholls J. G., Baylor D. A. Specific modalities and receptive fields of sensory neurons in CNS of the leech. J Neurophysiol. 1968 Sep;31(5):740–756. doi: 10.1152/jn.1968.31.5.740. [DOI] [PubMed] [Google Scholar]
- Ripley S. H., Bush B. M., Roberts A. Crab muscle receptor which responds without impulses. Nature. 1968 Jun 22;218(5147):1170–1171. doi: 10.1038/2181170a0. [DOI] [PubMed] [Google Scholar]
- Shaw S. R. Decremental conduction of the visual signal in barnacle lateral eye. J Physiol. 1972 Jan;220(1):145–175. doi: 10.1113/jphysiol.1972.sp009699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]