Abstract
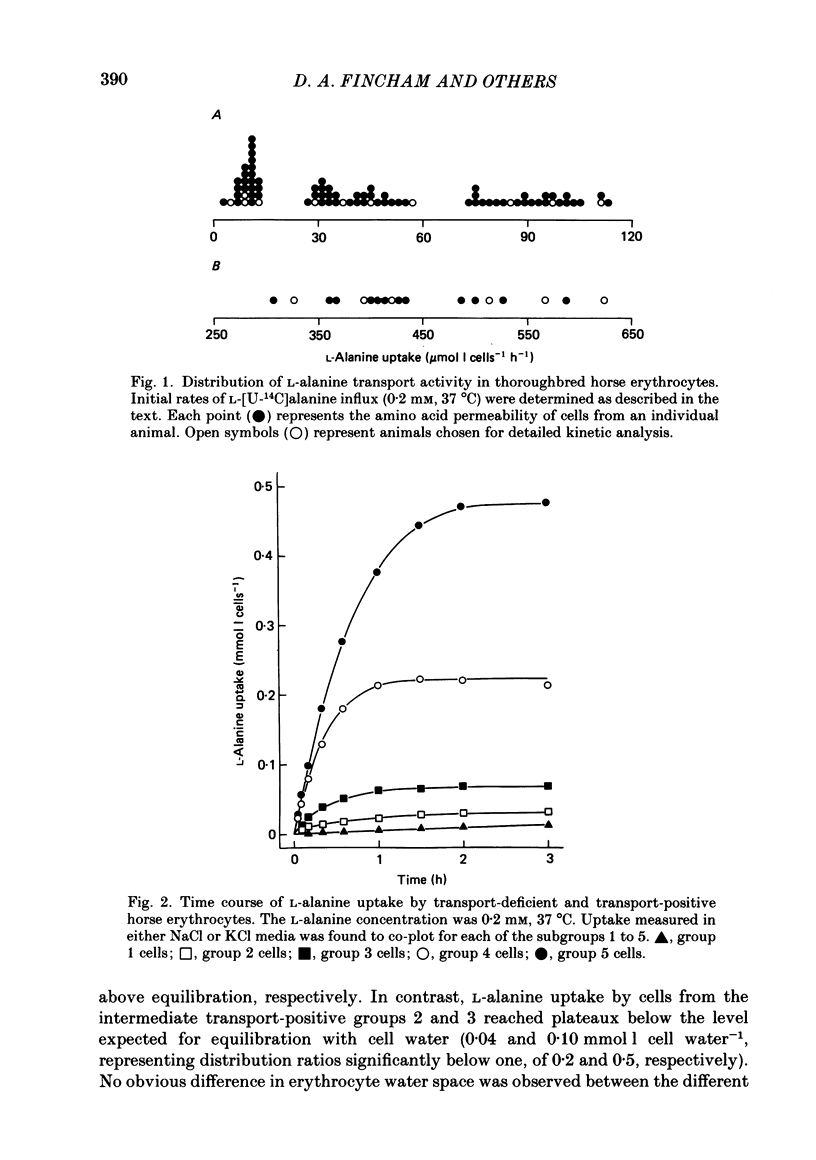
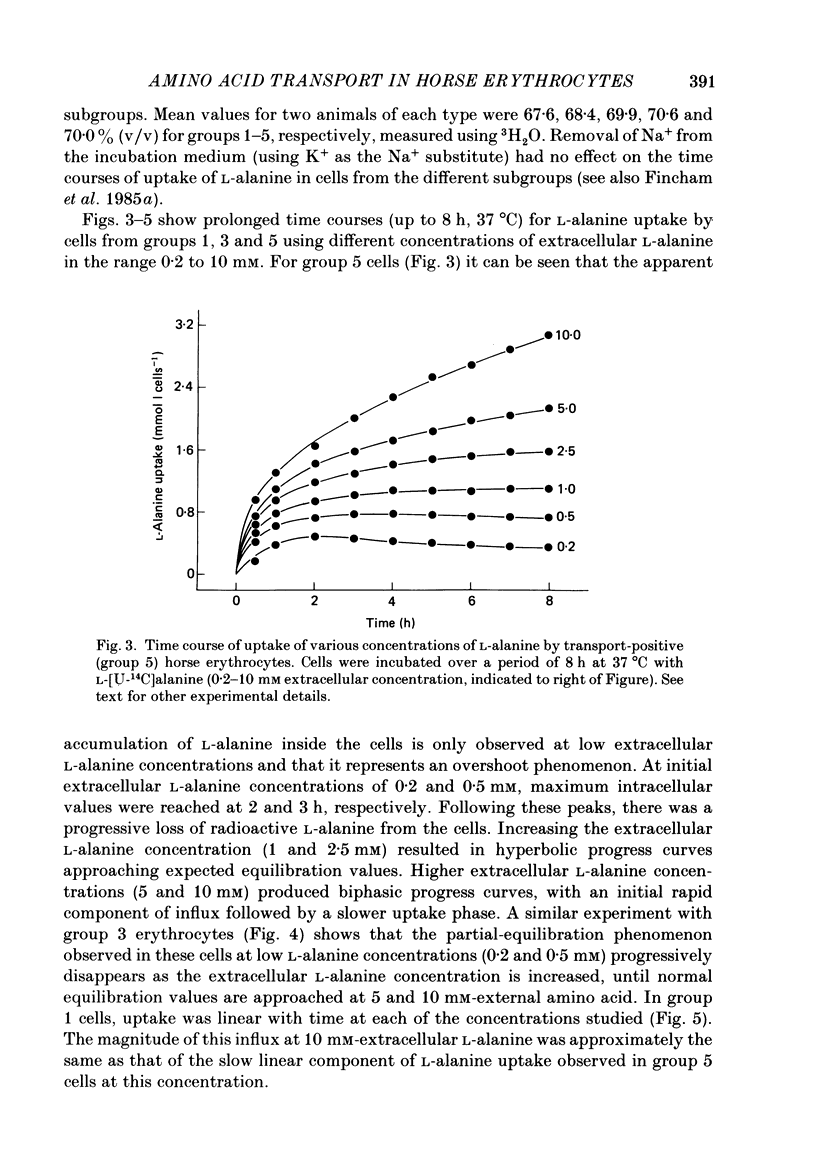
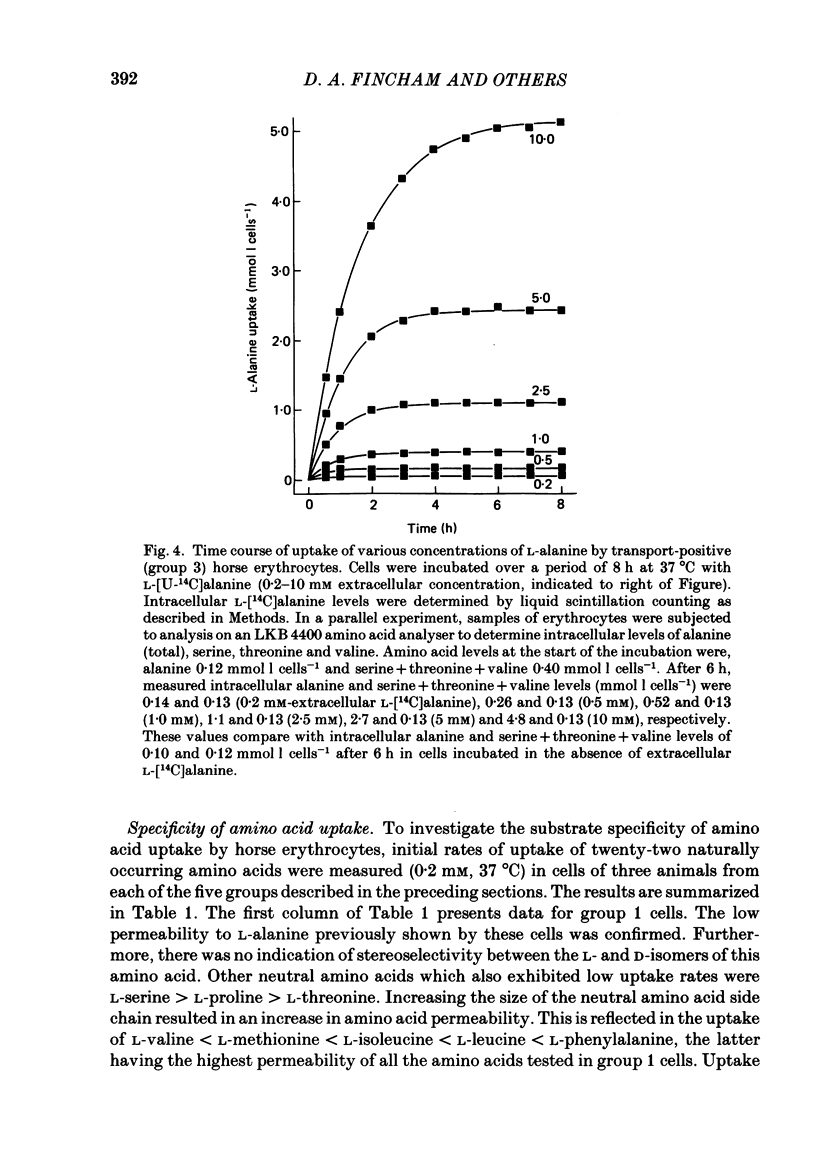
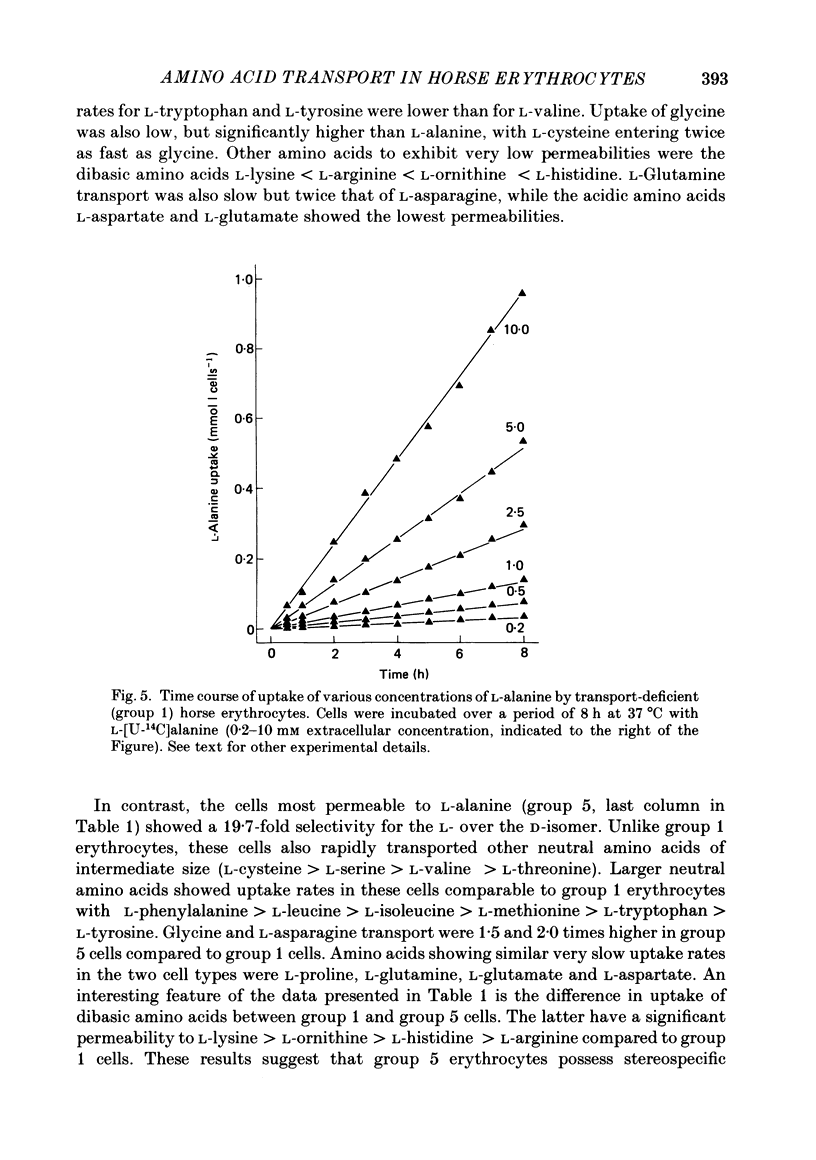
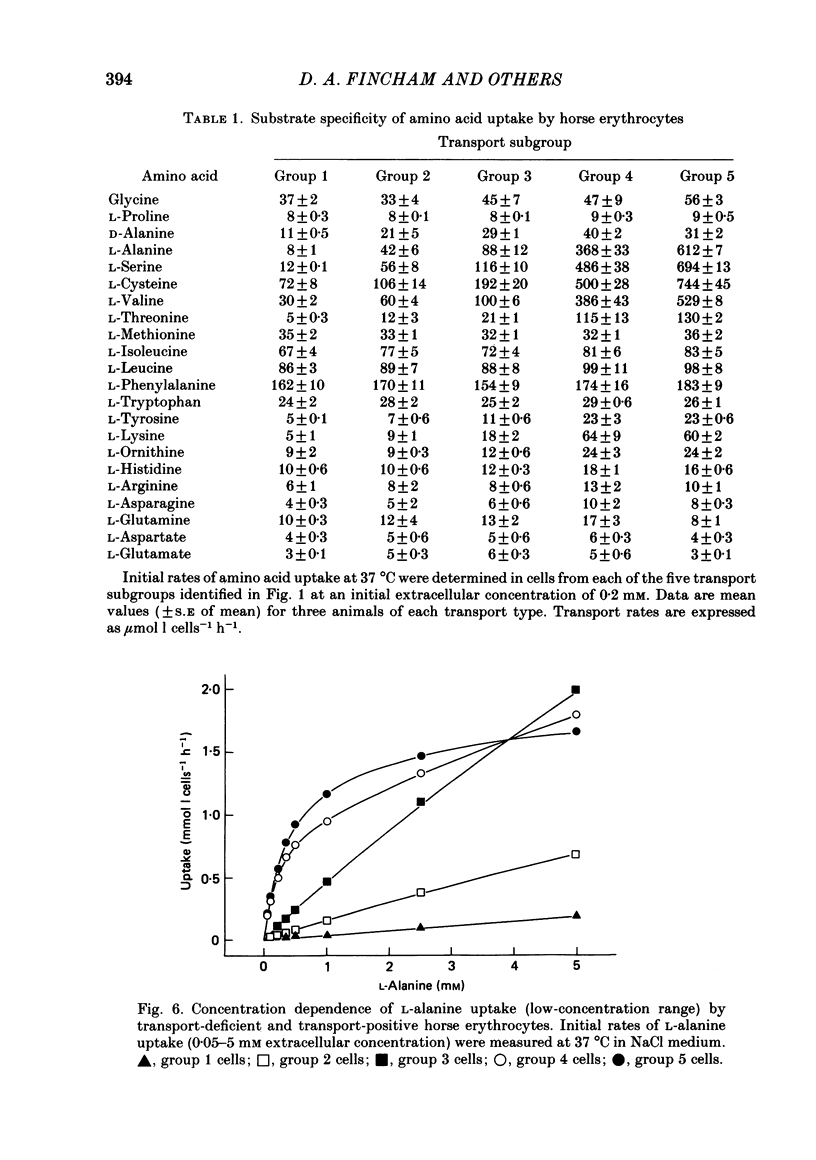
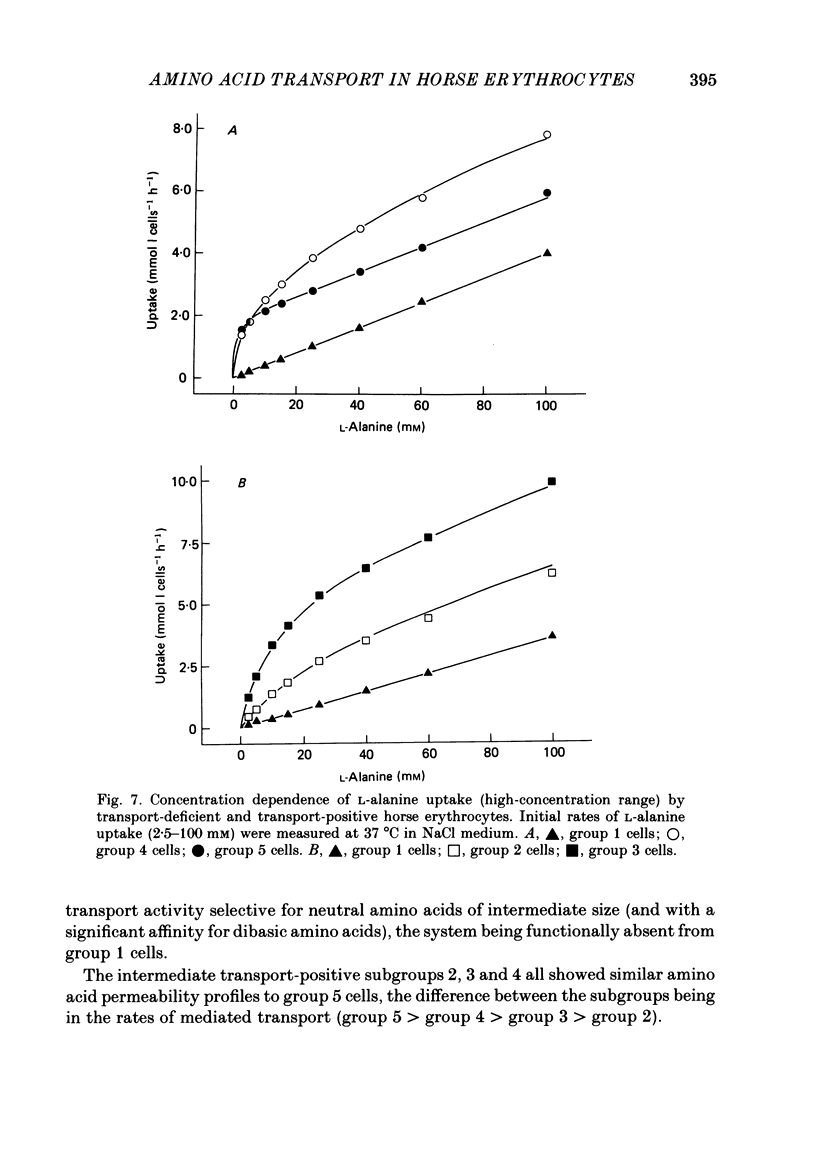
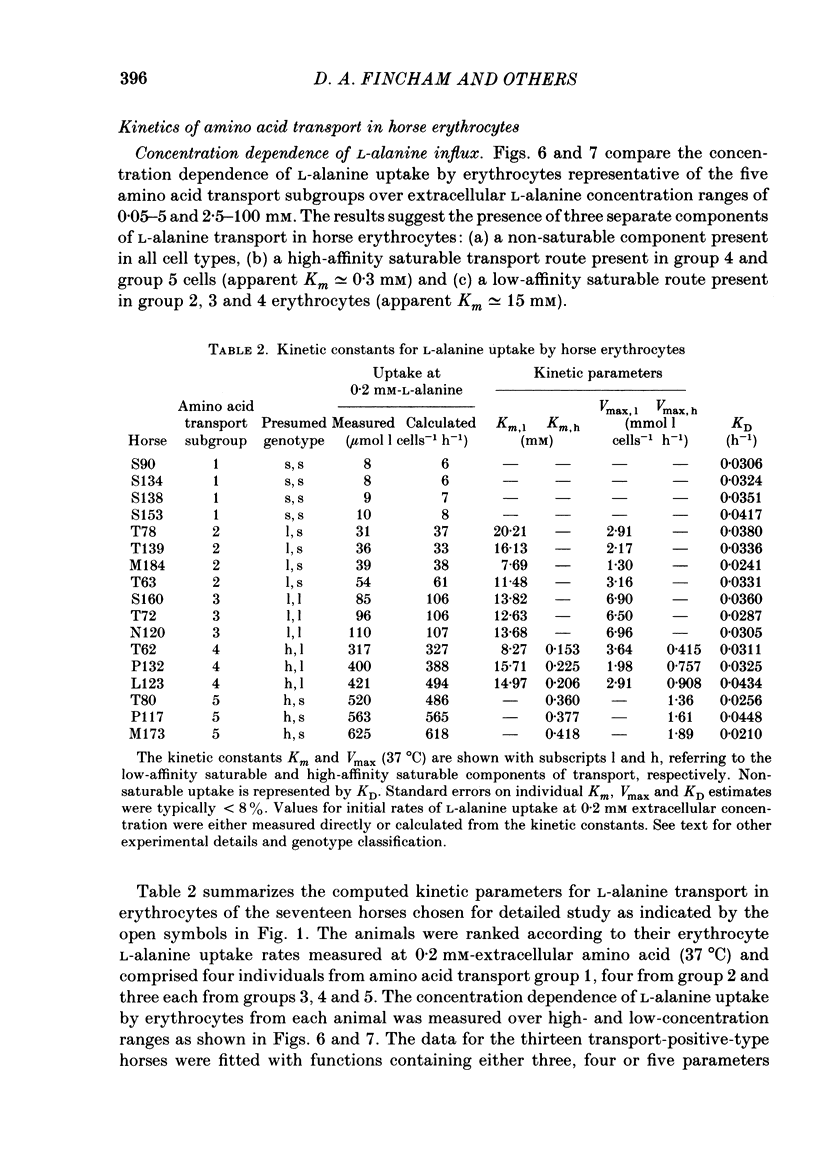
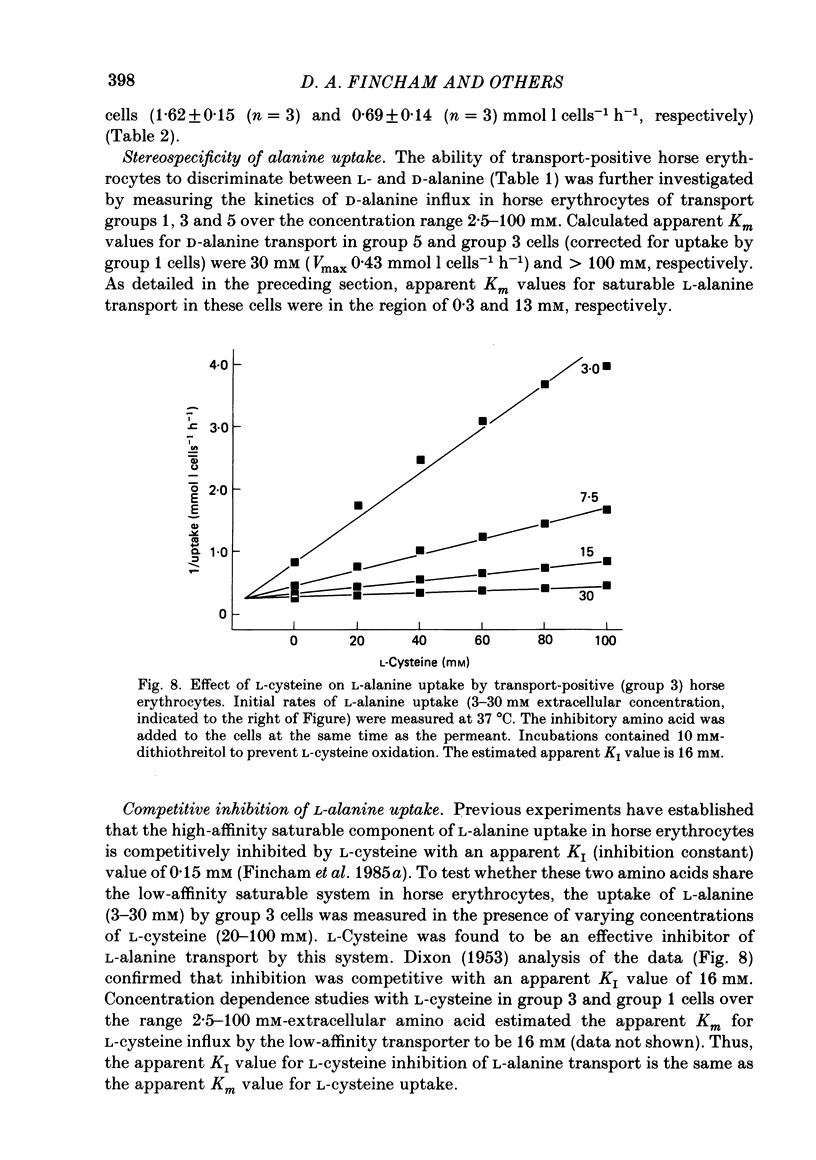
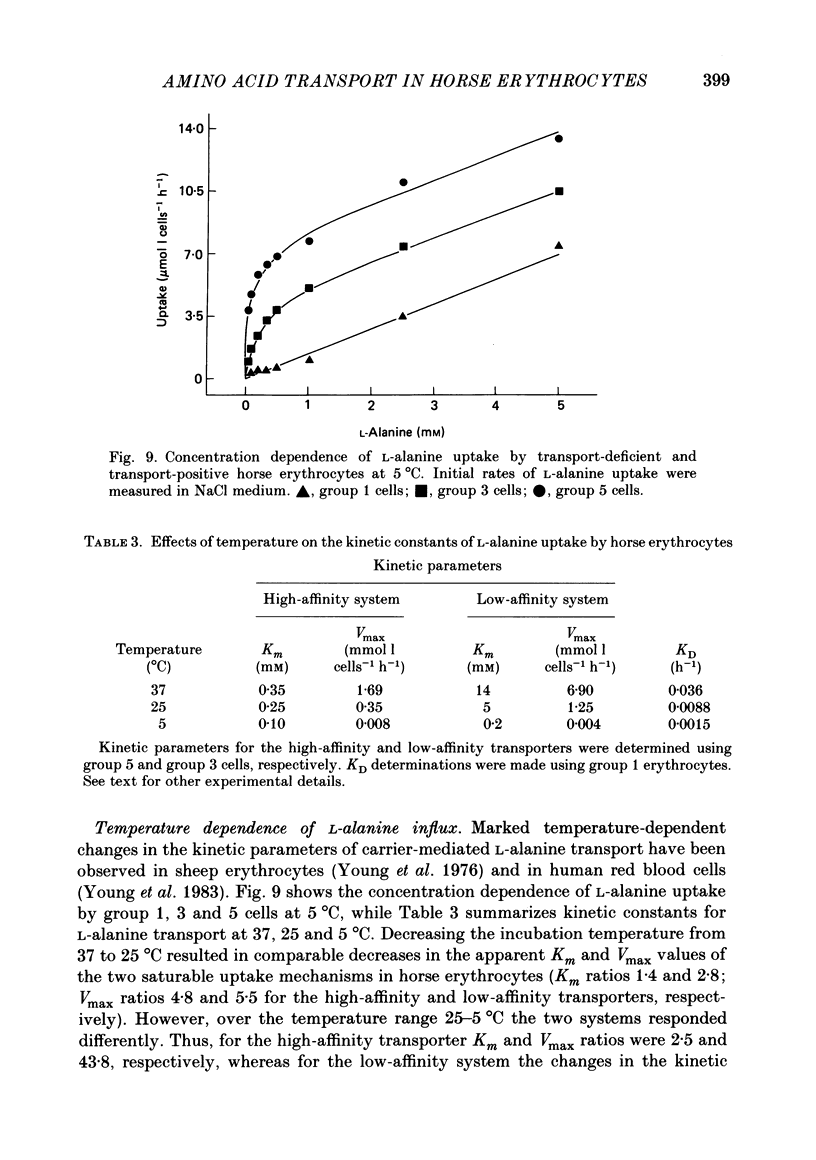
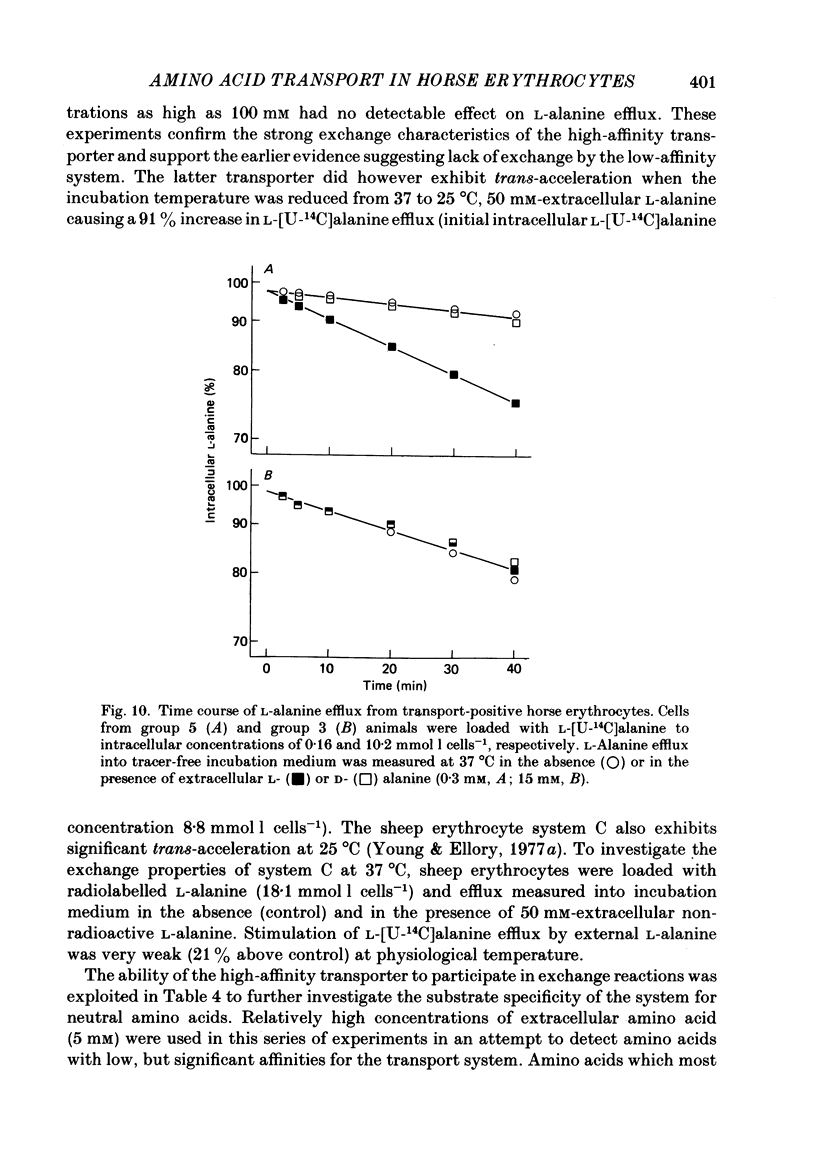
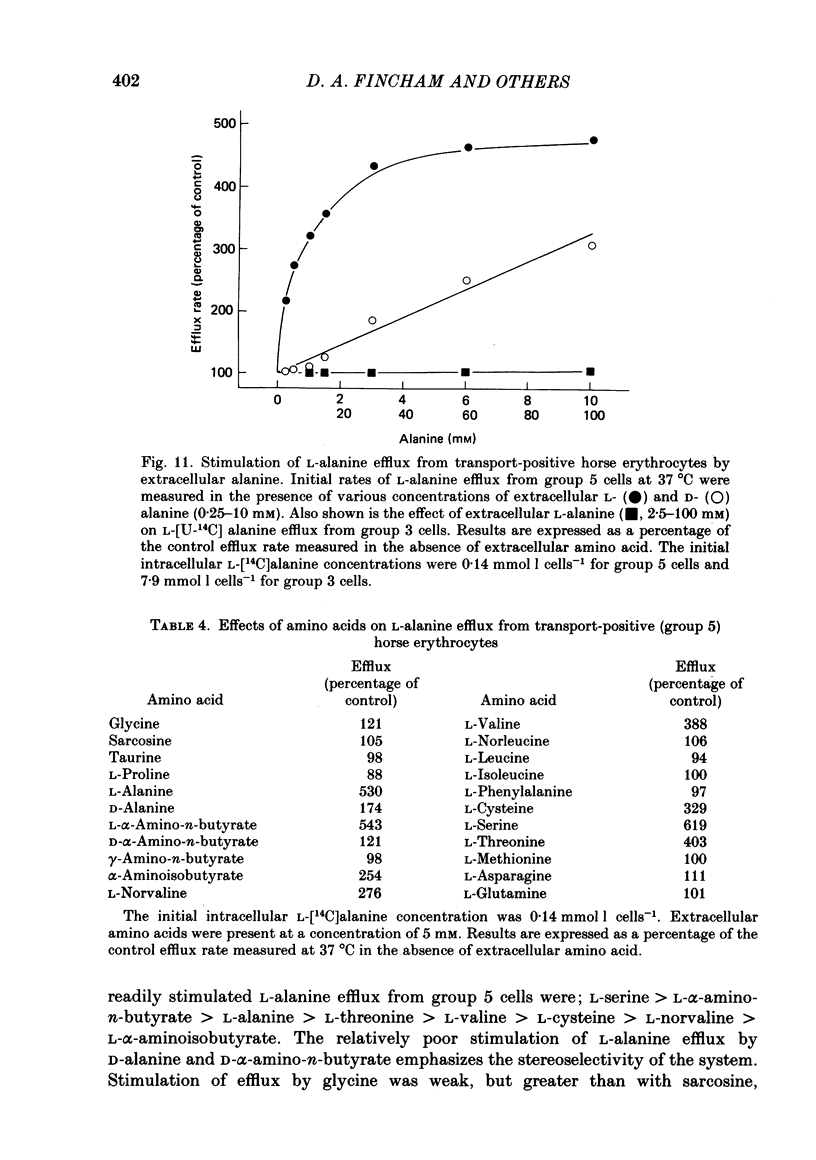
1. Thoroughbred horses were divisible into five distinct amino acid transport subgroups on the basis of their erythrocyte permeability to L-alanine, measured uptake rates ranging from 5 to 625 mumol l cells-1 h-1 (0.2 mM-extracellular L-alanine, 37 degrees C). 2. Erythrocytes from animals belonging to the lowest L-alanine permeability subgroup (5-15 mumol l cells-1 h-1) (transport-deficient type) exhibited slow nonsaturable transport of this amino acid. In contrast, cells from horses of the four transport-positive subgroups possessed additional high-affinity (apparent L-alanine Km (Michaelis constant) congruent to 0.3 mM) and/or low-affinity (apparent L-alanine Km congruent to 13 mM) Na+-independent transport routes selective for L-neutral amino acids of intermediate size. The two transporters, designated systems asc1 and asc2, respectively, also possessed a significant affinity for dibasic amino acids. 3. Amino acid transport activity in horse erythrocytes behaved as if controlled by three co-dominant alleles (s, h and l), where s is a silent allele, and h and l code for the functional presence of systems asc1 and asc2, respectively. 4. At physiological temperature, system asc1 operated preferentially in an exchange mode. In contrast, system asc2 did not participate in exchange reactions at 37 degrees C, but did exhibit significant trans-acceleration at 25 degrees C. 5. Reduction of the incubation temperature also resulted in dramatic decreases in apparent Km and Vmax for L-alanine uptake by system asc2, whereas the effects of temperature on system asc1 were much less marked. At 5 degrees C the two transporters exhibited equivalent kinetic constants for L-alanine influx. L-Alanine uptake by transport-deficient cells was relatively insensitive to temperature. Influx by this route may represent the ground-state permeability of the lipid bilayer. 6. The effects of low temperature on system asc2 suggest a preferential impairment of the mobility of the unloaded carrier relative to that of the loaded transporter. Similarly, the different kinetic properties of systems asc1 and asc2 at physiological temperature are attributed to a difference in the mobilities of the empty carriers, this difference being minimized at 5 degrees C.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Saleh E. A., Wheeler K. P. Transport of neutral amino acids by human erythrocytes. Biochim Biophys Acta. 1982 Jan 22;684(2):157–171. doi: 10.1016/0005-2736(82)90001-3. [DOI] [PubMed] [Google Scholar]
- Atkins G. L. Tests for the goodness of fit of models. Biochem Soc Trans. 1976;4(2):357–361. doi: 10.1042/bst0040357. [DOI] [PubMed] [Google Scholar]
- Bannai S., Christensen H. N., Vadgama J. V., Ellory J. C., Englesberg E., Guidotti G. G., Gazzola G. C., Kilberg M. S., Lajtha A., Sacktor B. Amino acid transport systems. 1984 Sep 27-Oct 3Nature. 311(5984):308–308. doi: 10.1038/311308b0. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Ginsburg H. Transport of uridine in human red blood cells. Demonstration of a simple carrier-mediated process. J Gen Physiol. 1977 Jan;69(1):75–96. doi: 10.1085/jgp.69.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H. N. Exploiting amino acid structure to learn about membrane transport. Adv Enzymol Relat Areas Mol Biol. 1979;49:41–101. doi: 10.1002/9780470122945.ch2. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. Organic ion transport during seven decades. The amino acids. Biochim Biophys Acta. 1984 Sep 3;779(3):255–269. doi: 10.1016/0304-4157(84)90012-1. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellory J. C., Jones S. E., Young J. D. Glycine transport in human erythrocytes. J Physiol. 1981 Nov;320:403–422. doi: 10.1113/jphysiol.1981.sp013958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellory J. C., Tucker E. M., Deverson E. V. The identification of ornithine and lysine at high concentrations in the red cells of sheep with an inherited deficiency of glutathione. Biochim Biophys Acta. 1972 Oct 25;279(3):481–483. doi: 10.1016/0304-4165(72)90169-9. [DOI] [PubMed] [Google Scholar]
- Fincham D. A., Mason D. K., Young J. D. Characterization of a novel Na+-independent amino acid transporter in horse erythrocytes. Biochem J. 1985 Apr 1;227(1):13–20. doi: 10.1042/bj2270013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham D. A., Young J. D., Mason D. K., Collins E. A., Snow D. H. Breed and species comparison of amino acid transport variation in equine erythrocytes. Res Vet Sci. 1985 May;38(3):346–351. [PubMed] [Google Scholar]
- Gardner J. D., Levy A. G. Transport of dibasic amino acids by human erythrocytes. Metabolism. 1972 May;21(5):413–431. doi: 10.1016/0026-0495(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Hoare D. G. The temperature dependence of the transport of L-leucine in human erythrocytes. J Physiol. 1972 Mar;221(2):331–348. doi: 10.1113/jphysiol.1972.sp009754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare D. G. The transport of L-leucine in human erythrocytes: a new kinetic analysis. J Physiol. 1972 Mar;221(2):311–329. doi: 10.1113/jphysiol.1972.sp009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba M., Maede Y. Increase of Na+ gradient-dependent L-glutamate and L-aspartate transport in high K+ dog erythrocytes associated with high activity of (Na+, K+)-ATPase. J Biol Chem. 1984 Jan 10;259(1):312–317. [PubMed] [Google Scholar]
- Jarvis S. M., Martin B. W. Effects of temperature on the transport of nucleosides in guinea pig erythrocytes. Can J Physiol Pharmacol. 1986 Feb;64(2):193–198. doi: 10.1139/y86-029. [DOI] [PubMed] [Google Scholar]
- King G. F., Kuchel P. W. A proton n.m.r. study of iminodipeptide transport and hydrolysis in the human erythrocyte. Possible physiological roles for the coupled system. Biochem J. 1984 Jun 1;220(2):553–560. doi: 10.1042/bj2200553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. F., Kuchel P. W. Assimilation of alpha-glutamyl-peptides by human erythrocytes. A possible means of glutamate supply for glutathione synthesis. Biochem J. 1985 May 1;227(3):833–842. doi: 10.1042/bj2270833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. F., York M. J., Chapman B. E., Kuchel P. W. Proton NMR spectroscopic studies of dipeptidase in human erythrocytes. Biochem Biophys Res Commun. 1983 Jan 14;110(1):305–312. doi: 10.1016/0006-291x(83)91296-2. [DOI] [PubMed] [Google Scholar]
- Lieb W. R., Stein W. D. Testing and characterizing the simple carrier. Biochim Biophys Acta. 1974 Dec 10;373(2):178–196. doi: 10.1016/0005-2736(74)90144-8. [DOI] [PubMed] [Google Scholar]
- Maede Y., Inaba M., Taniguchi N. Increase of Na-K-ATPase activity, glutamate, and aspartate uptake in dog erythrocytes associated with hereditary high accumulation of GSH, glutamate, glutamine, and aspartate. Blood. 1983 Mar;61(3):493–499. [PubMed] [Google Scholar]
- Plagemann P. G., Wohlhueter R. M. Effect of temperature on kinetics and differential mobility of empty and loaded nucleoside transporter of human erythrocytes. J Biol Chem. 1984 Jul 25;259(14):9024–9027. [PubMed] [Google Scholar]
- Rosenberg R. A kinetic analysis of L-tryptophan transport in human red blood cells. Biochim Biophys Acta. 1981 Dec 7;649(2):262–268. doi: 10.1016/0005-2736(81)90414-4. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. L-Leucine transport in human red blood cells: a detailed kinetic analysis. J Membr Biol. 1981;62(1-2):79–93. doi: 10.1007/BF01870202. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. Na-independent and Na-dependent transport of neutral amino acids in the human red blood cell. Acta Physiol Scand. 1982 Dec;116(4):321–330. doi: 10.1111/j.1748-1716.1982.tb07149.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg R., Young J. D., Ellory J. C. L-Tryptophan transport in human red blood cells. Biochim Biophys Acta. 1980 May 23;598(2):375–384. doi: 10.1016/0005-2736(80)90015-2. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Christensen H. N. Indications of spatial relations among structures recognizing amino acids and Na+ at a transport receptor site. Biochem Biophys Res Commun. 1970 Jul 27;40(2):277–283. doi: 10.1016/0006-291x(70)91006-5. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Christensen H. N. Nature of the cosubstrate action of Na+ and neutral amino acids in a transport system. J Biol Chem. 1971 Mar 25;246(6):1682–1688. [PubMed] [Google Scholar]
- Tucker E. M. A shortened life span of sheep red cells with a glutathione deficiency. Res Vet Sci. 1974 Jan;16(1):19–22. [PubMed] [Google Scholar]
- Tucker E. M., Wright P. C., Young J. D. Influence of arginase deficiency on amino acid concentrations in sheep erythrocytes with a normal and with a defective transport system for amino acids [proceedings]. J Physiol. 1977 Oct;271(2):47P–48P. [PubMed] [Google Scholar]
- Tucker E. M., Young J. D. Biochemical changes during reticulocyte maturation in culture. A comparison of genetically different sheep erythrocytes. Biochem J. 1980 Oct 15;192(1):33–39. doi: 10.1042/bj1920033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker E. M., Young J. D., Crowley C. Red cell glutathione deficiency: clinical and biochemical investigations using sheep as an experimental model system. Br J Haematol. 1981 Jul;48(3):403–415. doi: 10.1111/j.1365-2141.1981.tb02732.x. [DOI] [PubMed] [Google Scholar]
- Vadgama J. V., Christensen H. N. A new Na+-independent transport system for dipolar amino acids apparently corresponding to systems persisting after erythrocyte maturation in some mammalian genotypes. Ann N Y Acad Sci. 1985;456:454–456. doi: 10.1111/j.1749-6632.1985.tb14900.x. [DOI] [PubMed] [Google Scholar]
- Vadgama J. V., Christensen H. N. Discrimination of Na+-independent transport systems L, T, and asc in erythrocytes. Na+ independence of the latter a consequence of cell maturation? J Biol Chem. 1985 Mar 10;260(5):2912–2921. [PubMed] [Google Scholar]
- Vandenberg J. I., King G. F., Kuchel P. W. The assimilation of tri- and tetrapeptides by human erythrocytes. Biochim Biophys Acta. 1985 Jul 30;846(1):127–134. doi: 10.1016/0167-4889(85)90118-1. [DOI] [PubMed] [Google Scholar]
- WINTER C. G., CHRISTENSEN H. N. MIGRATION OF AMINO ACIDS ACROSS THE MEMBRANE OF THE HUMAN ERYTHROCYTE. J Biol Chem. 1964 Mar;239:872–878. [PubMed] [Google Scholar]
- Winter C. G., Christensen H. N. Contrasts in neutral amino acid transport by rabbit erythrocytes and reticulocytes. J Biol Chem. 1965 Sep;240(9):3594–3600. [PubMed] [Google Scholar]
- Young J. D., Ellory J. C. Substrate specificity of amino acid transport in sheep erythrocytes. Biochem J. 1977 Jan 15;162(1):33–38. doi: 10.1042/bj1620033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Ellory J. C., Tucker E. M. Amino acid transport defect in glutathione-deficient sheep erythrocytes. Nature. 1975 Mar 13;254(5496):156–157. doi: 10.1038/254156a0. [DOI] [PubMed] [Google Scholar]
- Young J. D., Ellory J. C., Tucker E. M. Amino acid transport in normal and glutathione-deficient sheep erythrocytes. Biochem J. 1976 Jan 15;154(1):43–48. doi: 10.1042/bj1540043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Jones S. E., Ellory J. C. Amino acid transport in human and in sheep erythrocytes. Proc R Soc Lond B Biol Sci. 1980 Sep 26;209(1176):355–375. doi: 10.1098/rspb.1980.0100. [DOI] [PubMed] [Google Scholar]
- Young J. D., Jones S. E., Ellory J. C. Amino acid transport via the red cell anion transport system. Biochim Biophys Acta. 1981 Jul 6;645(1):157–160. doi: 10.1016/0005-2736(81)90524-1. [DOI] [PubMed] [Google Scholar]
- Young J. D., Tucker E. M., Kilgour L. Genetic control of amino acid transport in sheep erythrocytes. Biochem Genet. 1982 Aug;20(7-8):723–731. doi: 10.1007/BF00483969. [DOI] [PubMed] [Google Scholar]
- Young J. D., Wolowyk M. W., Jones S. E., Ellory J. C. Sodium-dependent cysteine transport in human red blood cells. Nature. 1979 Jun 28;279(5716):800–802. doi: 10.1038/279800a0. [DOI] [PubMed] [Google Scholar]
- Young J. D., Wolowyk M. W., Jones S. M., Ellory J. C. Red-cell amino acid transport. Evidence for the presence of system ASC in mature human red blood cells. Biochem J. 1983 Nov 15;216(2):349–357. doi: 10.1042/bj2160349. [DOI] [PMC free article] [PubMed] [Google Scholar]


