Abstract
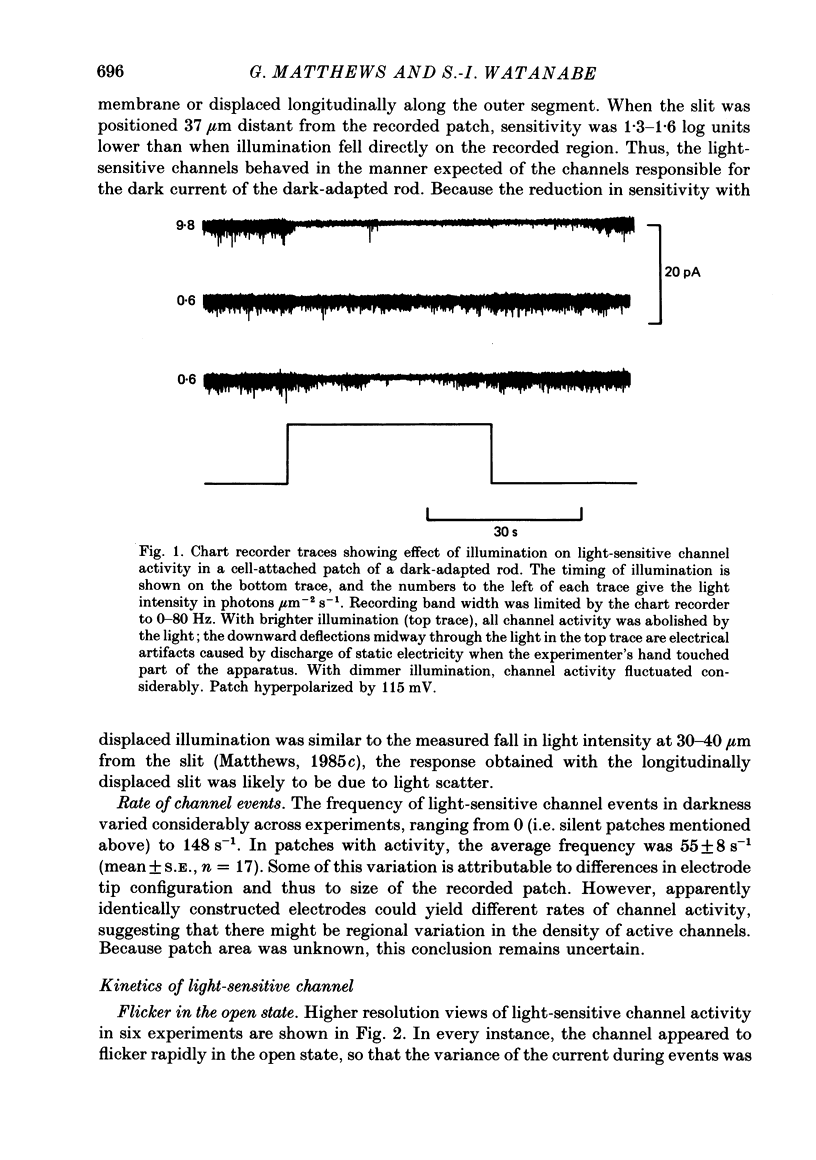
1. In patch-clamp recordings from outer segments of dark-adapted rod photoreceptors, single-channel recordings were obtained from the light-sensitive conductance when divalent cations were omitted from the pipette solution bathing the extracellular face of the recorded patch of membrane. 2. Activity of the light-sensitive channel was suppressed by light within the normal response range of the dark-adapted rod. During dim, steady illumination, the rate of opening of the channel fluctuated dramatically, as expected qualitatively from statistical fluctuations in the number of photoisomerizations occurring within the effective collecting area of the recorded patch. 3. The light-sensitive channel flickered rapidly in the open state, so that individual events appeared as a burst of openings and closings. The average duration of a burst was 0.78 +/- 0.03 ms (mean +/- S.E.). The average duration of an individual opening was 0.18 +/- 0.008 ms. The average closed duration within a burst was 0.37 +/- 0.02 ms. 4. Hyperpolarization of the recorded patch had no effect on average burst or open duration, although opening frequency increased slightly (+18.6 +/- 4.9%; n = 13; mean +/- S.E.). Average single-channel current increased linearly with hyperpolarization, giving an estimated single-channel conductance of 20.5 +/- 1.1 pS. By extrapolation of the relation between channel current and hyperpolarization, the dark driving force was estimated to be about 48 mV. 5. In addition to reducing the rate of channel events, dim non-saturating light also reduced the average duration of a burst of openings and the average duration of openings within a burst. 6. About 50% of cell-attached patches showed no channel activity in darkness. Light-suppressable channel activity could be induced in these silent patches by perfusing the outer segment with low-Ca2+ Ringer solution. Similarly, activity could be increased dramatically by low-Ca2+ Ringer solution in patches that did show channel activity in the dark. From the maximal channel activity observed during low-Ca2+ perfusion, the lower limit for the number of channels per patch was 20-70, corresponding to an estimated channel density of 100-350 channels micron-2. 7. After recording light-sensitive channel activity in the intact rod, the patch of membrane was excised, exposing the intracellular membrane face. Application of guanosine 3',5'-cyclic monophosphate (cyclic GMP) to the intracellular face activated channels (Haynes, Kay & Yau, 1986; Zimmerman & Baylor, 1986; Matthews, 1986d, 1987) whose properties could then be compared directly with the light-sensitive channels recorded earlier in the same patch of membrane.(ABSTRACT TRUNCATED AT 400 WORDS)
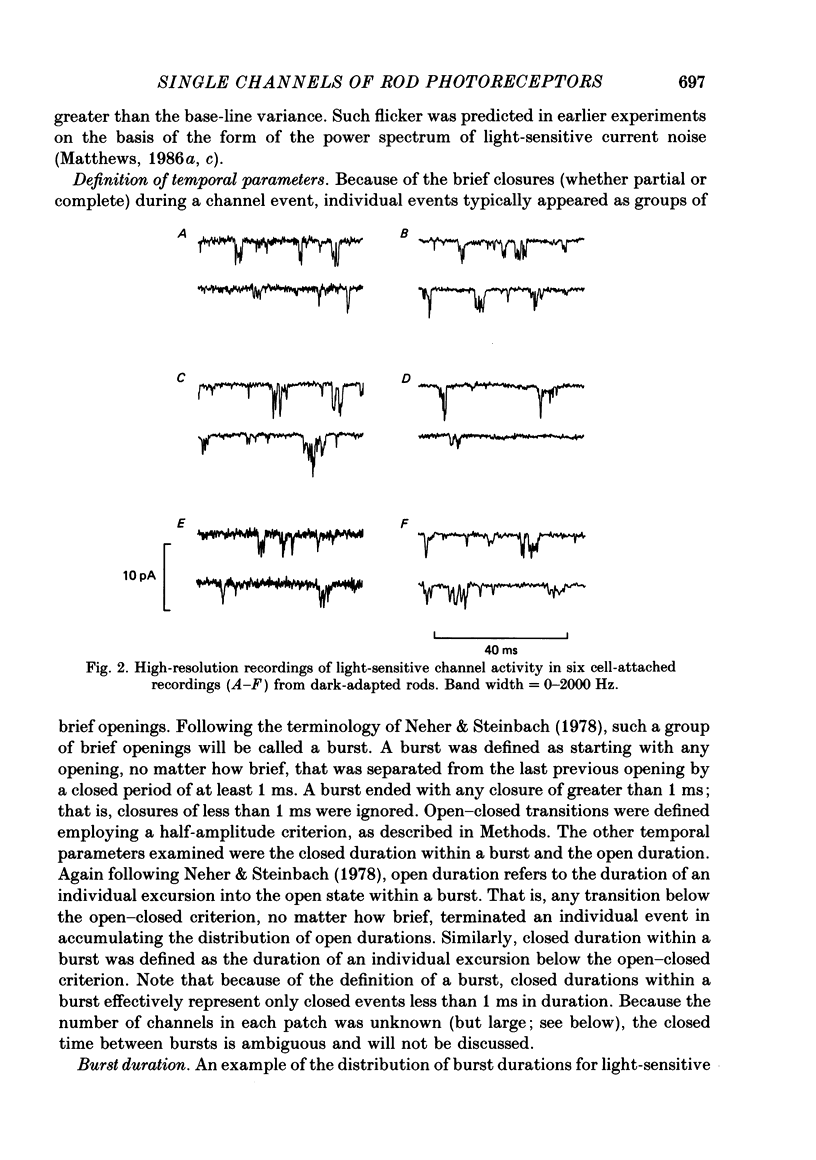
Full text
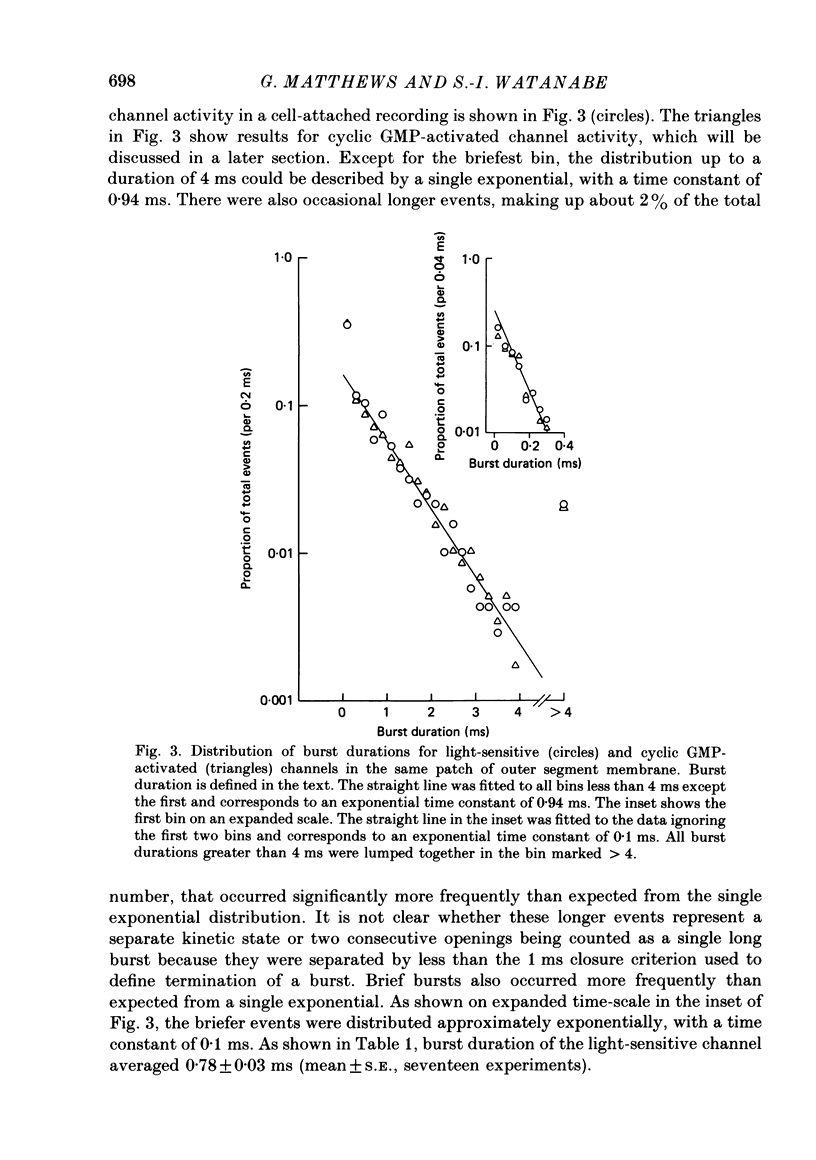
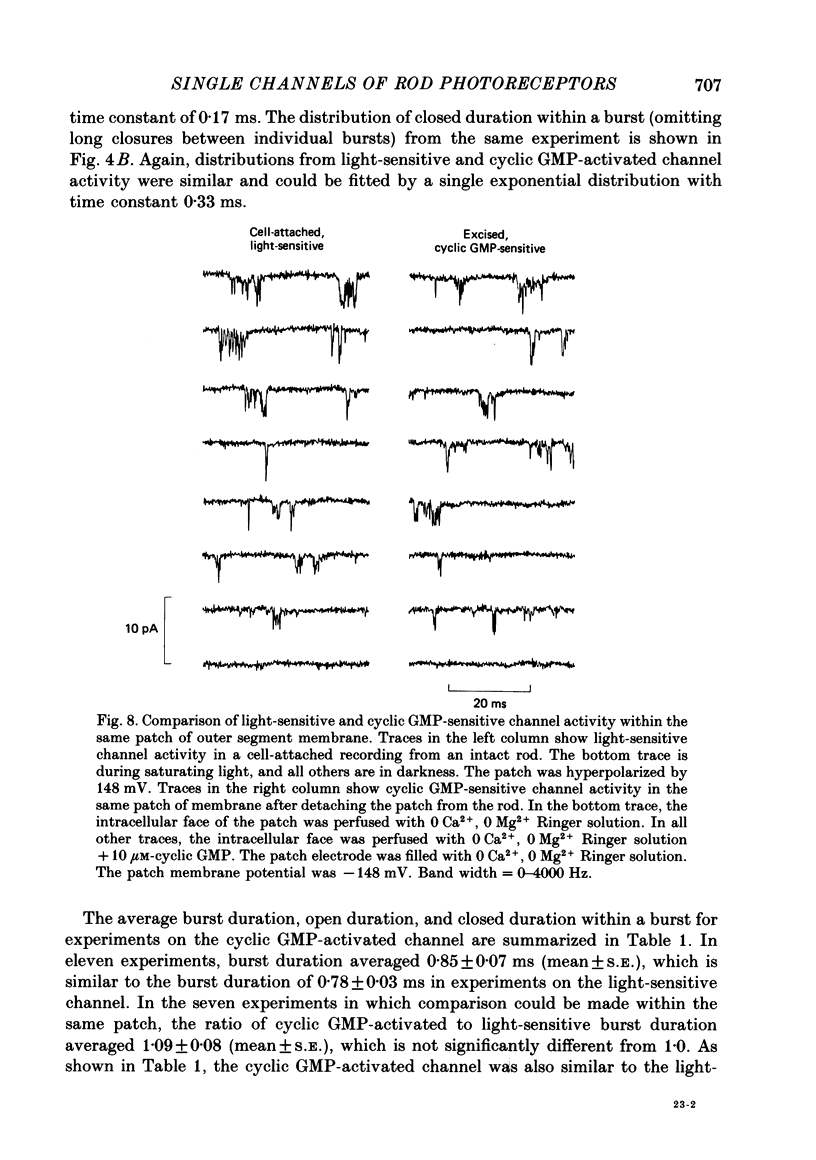
PDF




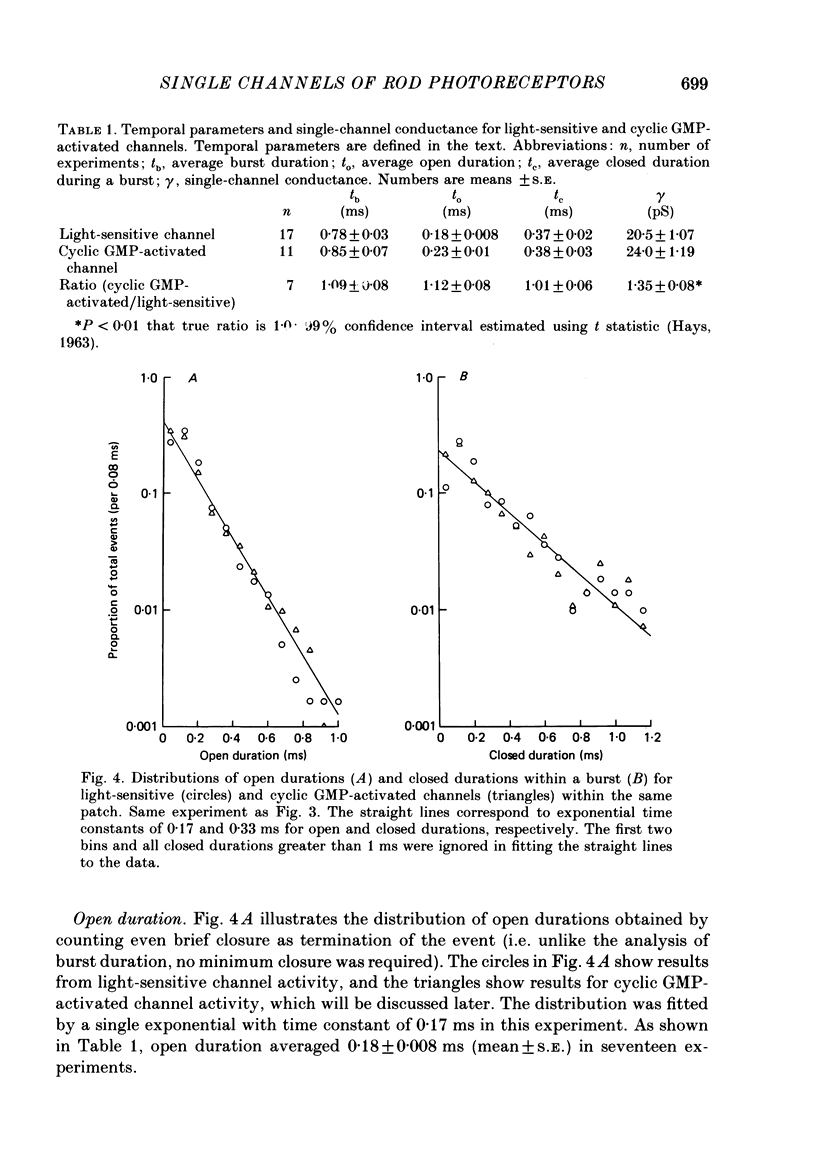
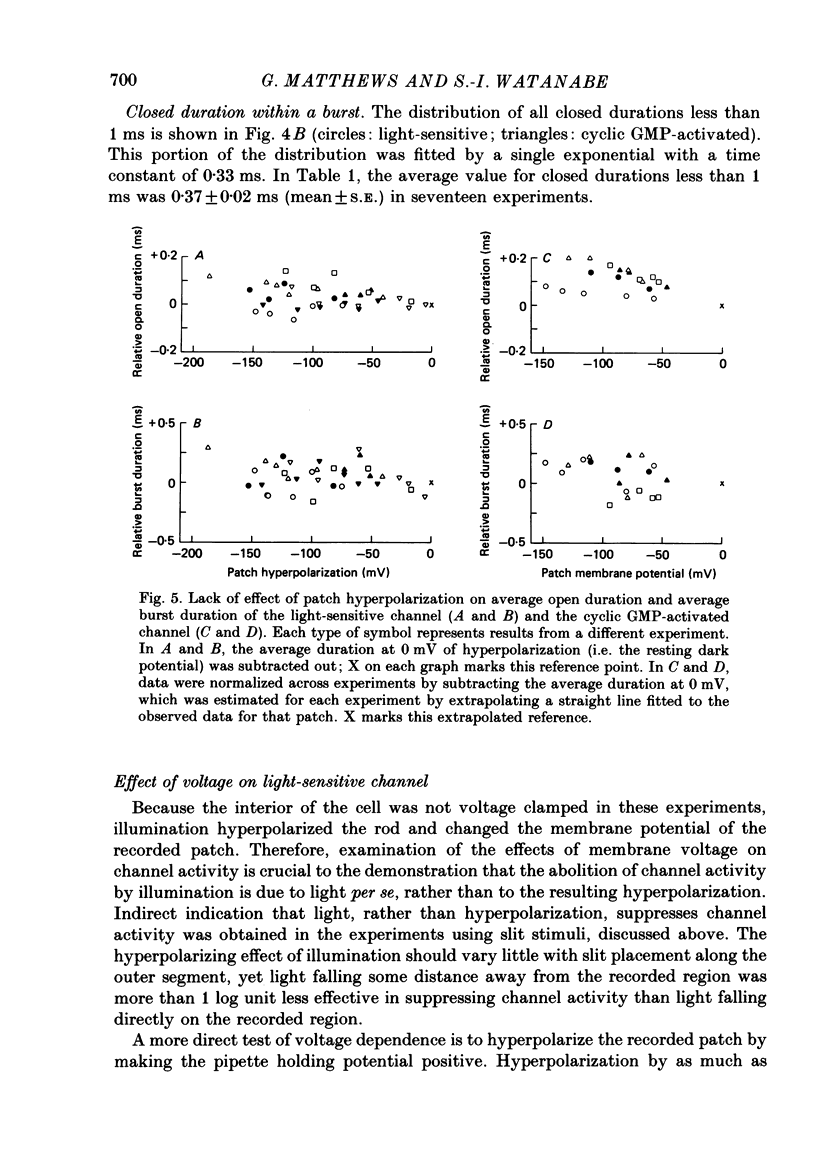
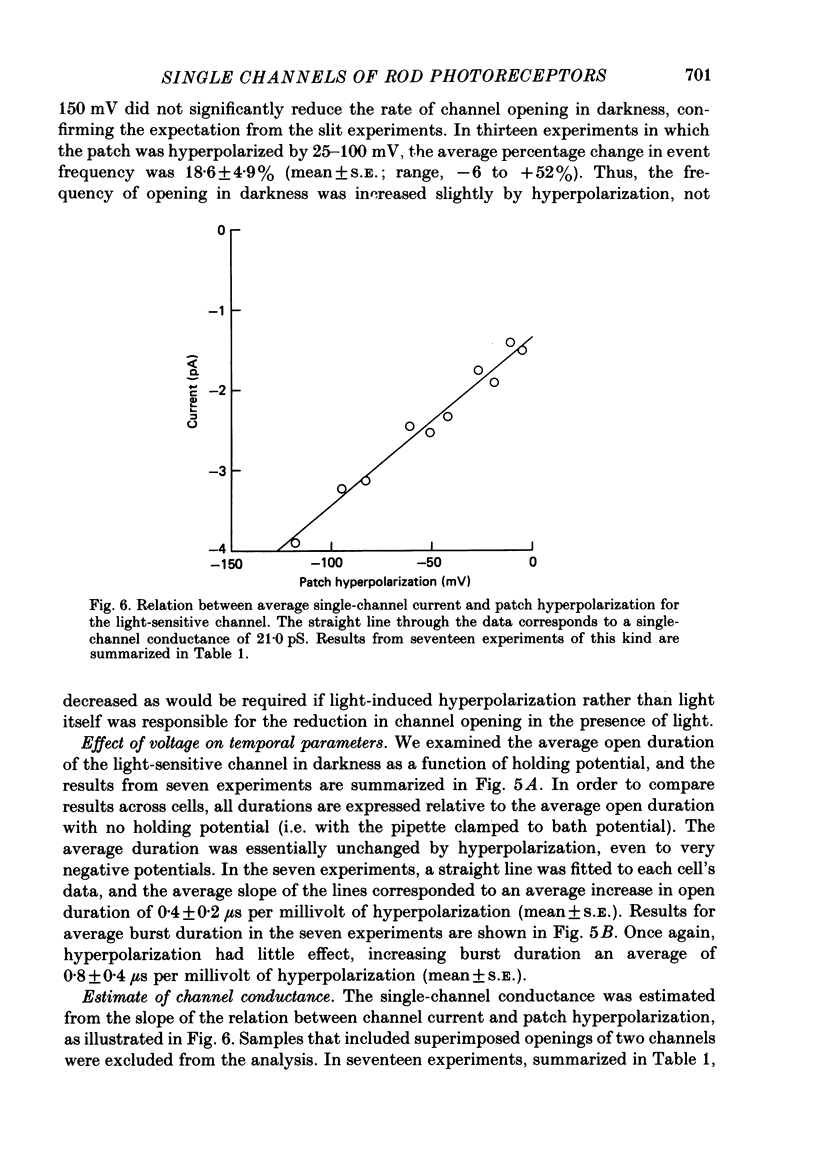









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
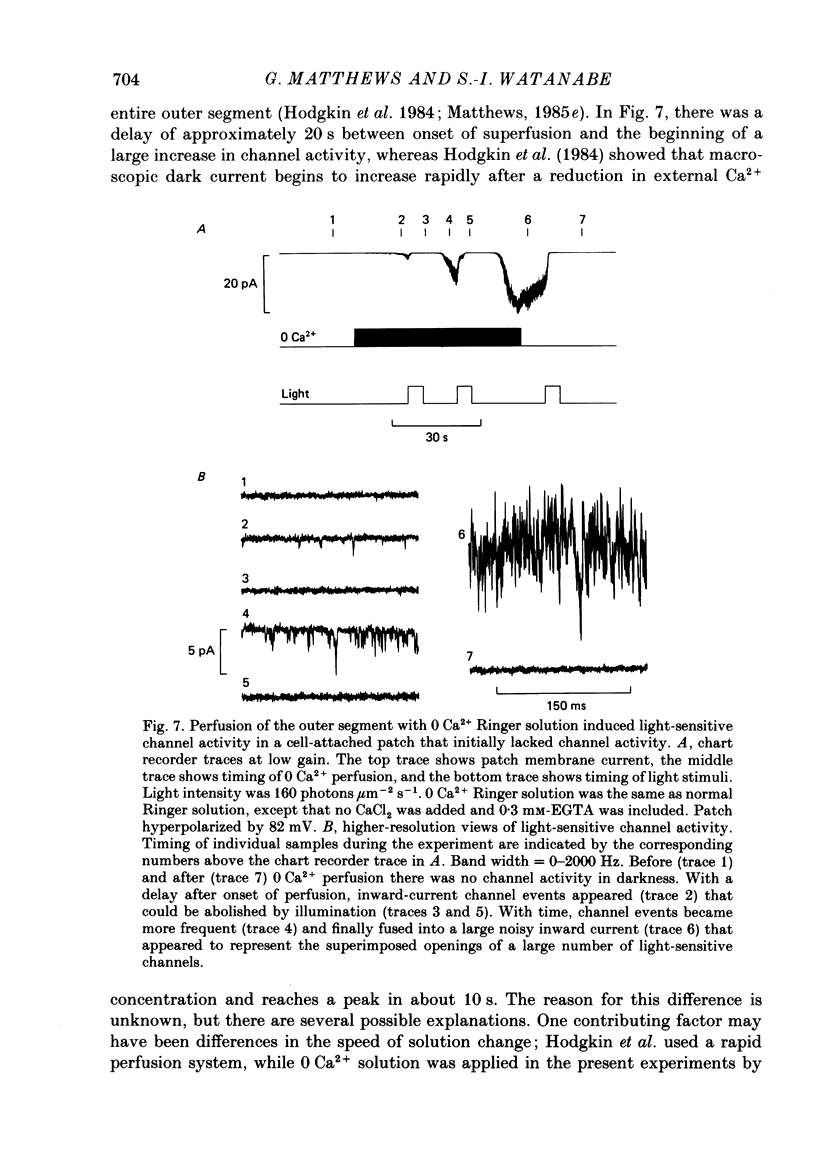
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J. Electrical properties of the light-sensitive conductance of rods of the salamander Ambystoma tigrinum. J Physiol. 1986 Feb;371:115–145. doi: 10.1113/jphysiol.1986.sp015964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodoia R. D., Detwiler P. B. Patch-clamp recordings of the light-sensitive dark noise in retinal rods from the lizard and frog. J Physiol. 1985 Oct;367:183–216. doi: 10.1113/jphysiol.1985.sp015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sheridan R. E. The modes of action of gallamine. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):181–203. doi: 10.1098/rspb.1981.0002. [DOI] [PubMed] [Google Scholar]
- Dekin M. S. Permeability changes induced by L-glutamate at the crayfish neuromuscular junction. J Physiol. 1983 Aug;341:105–125. doi: 10.1113/jphysiol.1983.sp014795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Gray P., Attwell D. Kinetics of light-sensitive channels in vertebrate photoreceptors. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):379–388. doi: 10.1098/rspb.1985.0007. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Haynes L. W., Kay A. R., Yau K. W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986 May 1;321(6065):66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Mueller P., Pugh E. N., Jr Protons suppress the dark current of frog retinal rods. J Physiol. 1984 Feb;347:85–110. doi: 10.1113/jphysiol.1984.sp015055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G. Comparison of the light-sensitive and cyclic GMP-sensitive conductances of the rod photoreceptor: noise characteristics. J Neurosci. 1986 Sep;6(9):2521–2526. doi: 10.1523/JNEUROSCI.06-09-02521.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G. Single-channel recordings demonstrate that cGMP opens the light-sensitive ion channel of the rod photoreceptor. Proc Natl Acad Sci U S A. 1987 Jan;84(1):299–302. doi: 10.1073/pnas.84.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G. Spatial spread of light-induced sensitization in rod photoreceptors exposed to low external calcium. Vision Res. 1985;25(6):733–740. doi: 10.1016/0042-6989(85)90180-4. [DOI] [PubMed] [Google Scholar]
- Matthews G. Spread of the light response along the rod outer segment: an estimate from patch-clamp recordings. Vision Res. 1986;26(4):535–541. doi: 10.1016/0042-6989(86)90002-7. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984 Oct 18;311(5987):661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986 May 1;321(6065):70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]


