Abstract
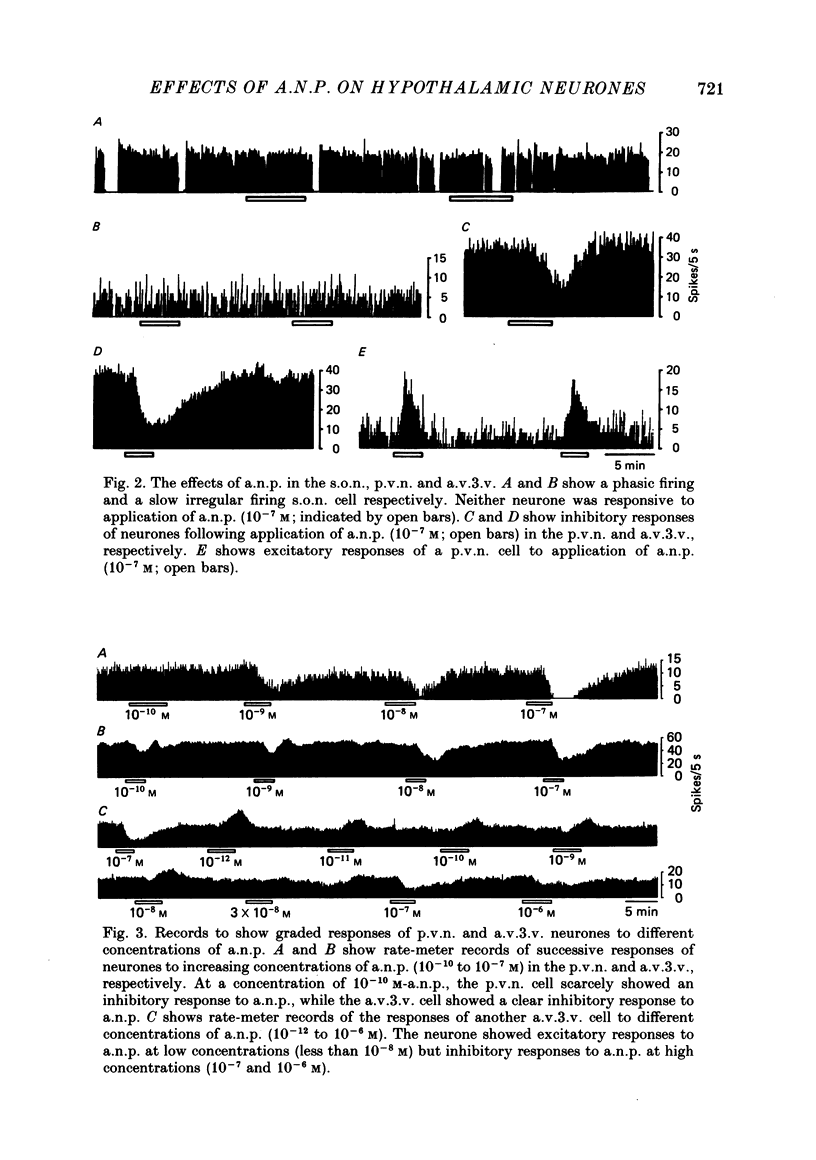
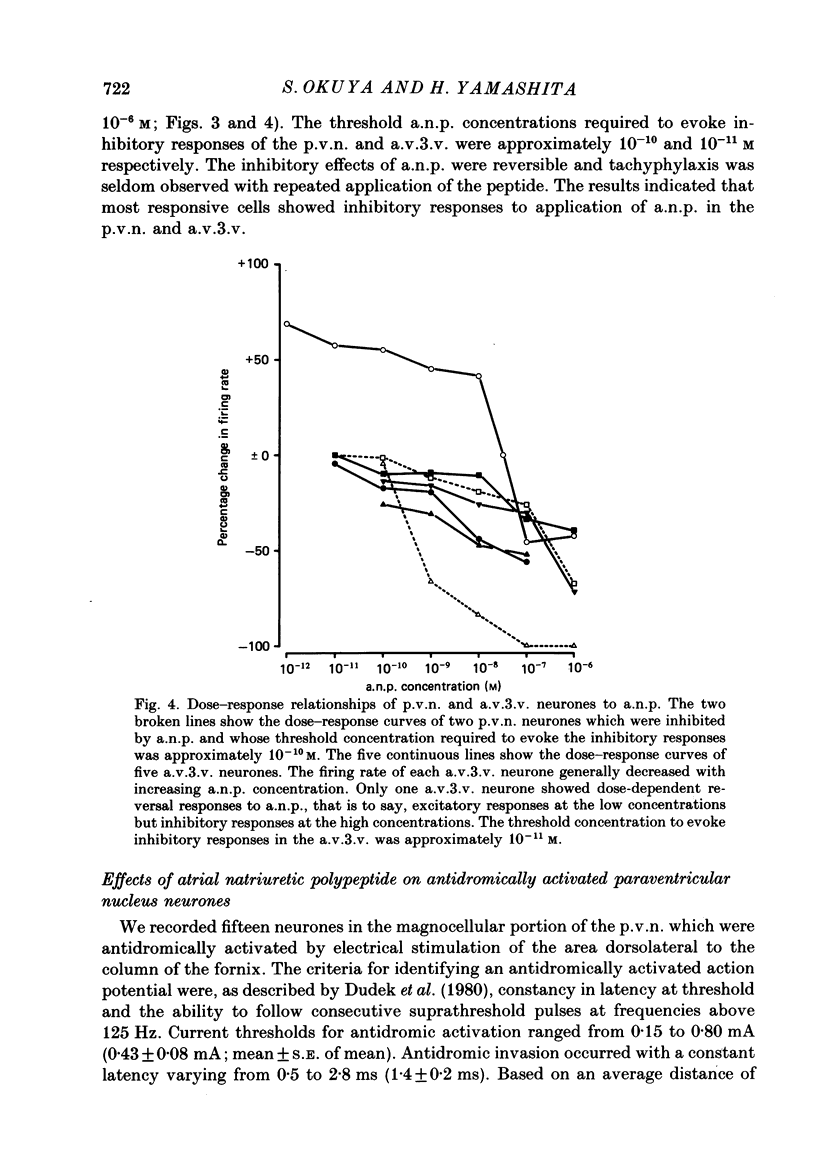
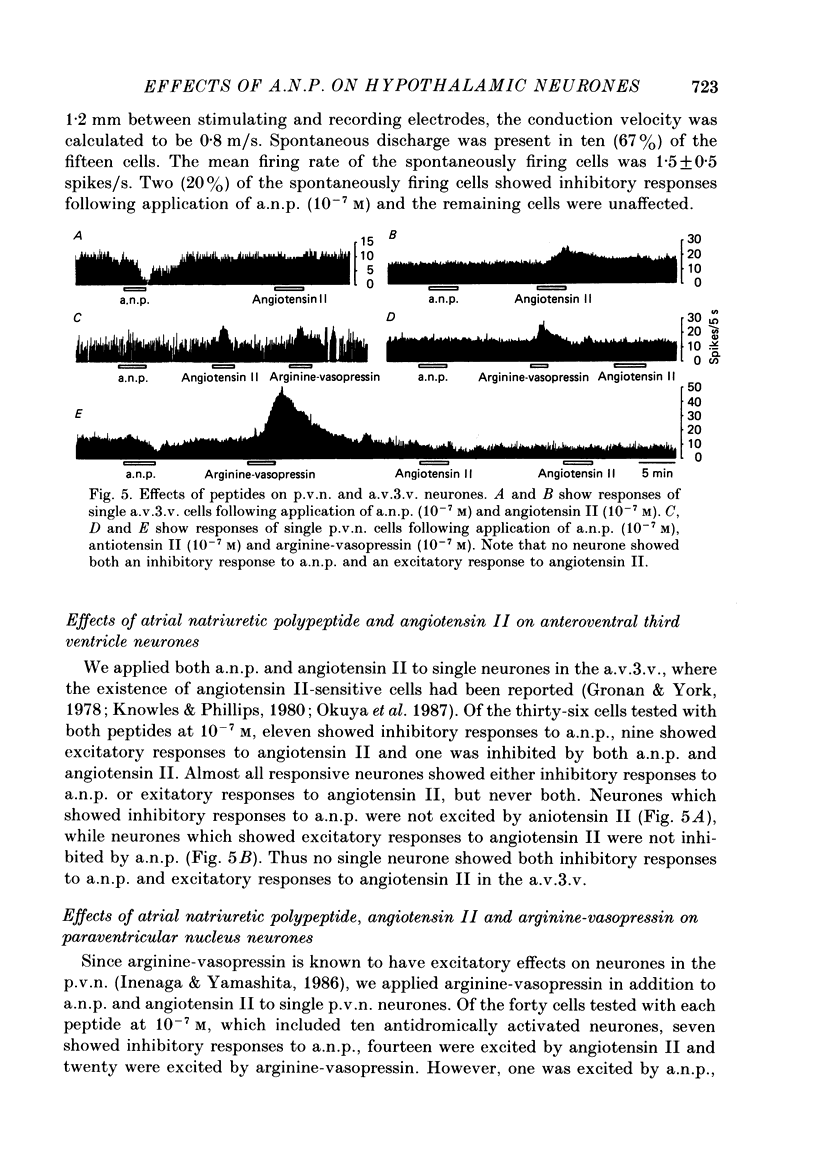
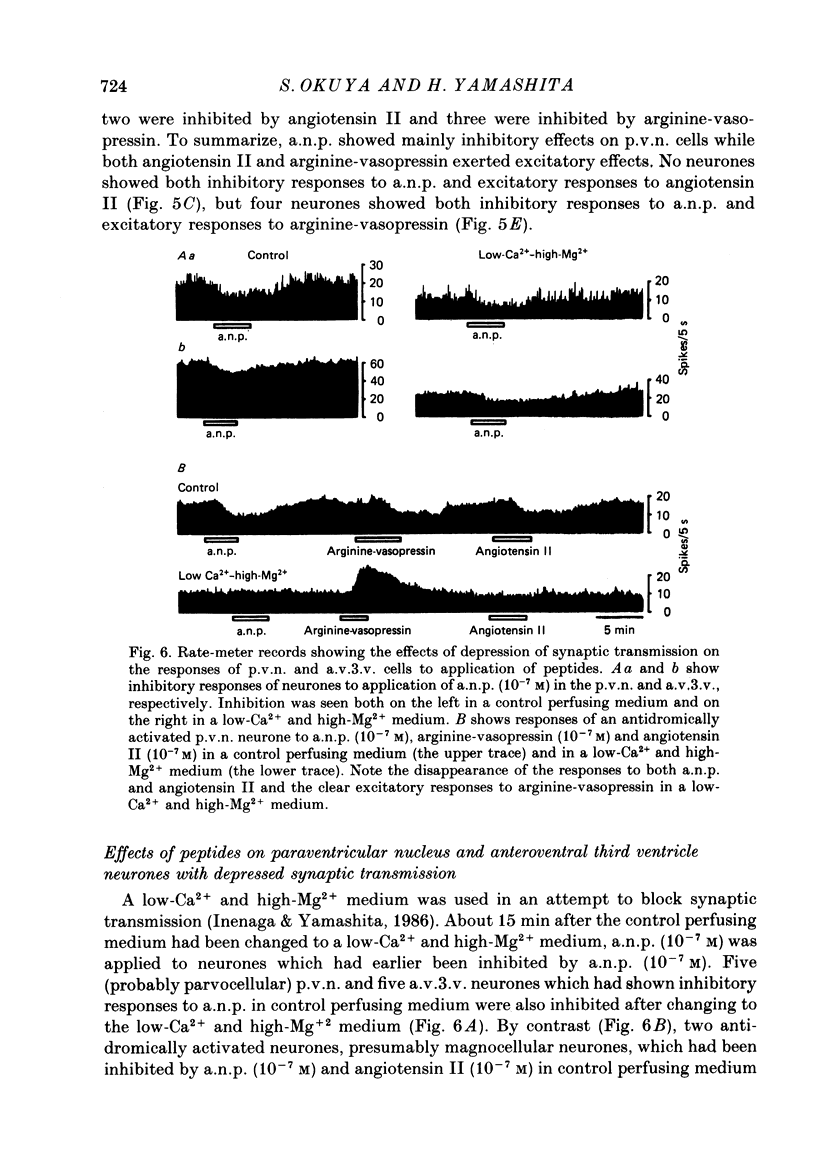
1. Extracellular recordings were made from 175 spontaneously active cells in the rat coronal hypothalamic slice preparation. Reconstruction of the recording sites showed that fifteen were in the supraoptic nucleus (s.o.n.), ten in the magnocellular portion of the paraventricular nucleus (p.v.n.) which could be antidromically activated by stimulation lateral to the nucleus, seventy-seven other cells in the p.v.n. and seventy-three in the anteroventral third ventricle (a.v.3.v.) region. 2. The mean firing rates (mean +/- S.E. of mean) of the spontaneously firing cells in the s.o.n., p.v.n. and a.v.3.v. were 2.8 +/- 0.4 spikes/s, 2.9 +/- 0.2 spikes/s and 5.0 +/- 0.4 spikes/s, respectively. Antidromically identified p.v.n. cells fired spontaneously with a mean firing rate of 1.5 +/- 0.5 spikes/s. 3. Bath application of atrial natriuretic polypeptide (a.n.p.; 10(-7) M) had no effect on fifteen s.o.n. cells tested but nineteen (22%) of eighty-seven p.v.n. cells (including two of the ten antidromically activated cells) and thirty (41%) of seventy-three a.v.3.v. cells showed inhibitory responses. Three (3%) cells in the p.v.n. were excited by a.n.p. 4. The dose dependence of the response to a.n.p. was tested in two p.v.n. and five a.v.3.v. cells. As a.n.p. concentration increased, the firing rates of all seven cells generally decreased. However, one a.v.3.v. neurone was excited at low concentrations (less than 10(-8) M) but inhibited at high concentrations (10(-7) and 10(-6) M) of a.n.p. The threshold concentration to evoke inhibitory responses in the p.v.n. was 10(-10) M and in the a.v.3.v. was 10(-11) M. 5. With the exception of the two antidromically activated p.v.n. cells, the inhibitory effect of a.n.p. still persisted after synaptic transmission had been suppressed with a low-Ca2+ and high-Mg2+ medium. 6. Thirty-six cells in the a.v.3.v. were tested with both a.n.p. and angiotensin II applied at 10(-7) M. Twelve showed inhibitory responses to a.n.p. and nine showed excitatory responses to angiotensin II. In other experiments, a.n.p., angiotensin II and arginine-vasopressin were each applied to neurones in the p.v.n. Of the forty cells tested with all three peptides at 10(-7) M, seven were inhibited by a.n.p., fourteen were excited by angiotensin II and twenty were excited by arginine-vasopressin. No neurones in either the p.v.n. or a.v.3.v. were inhibited by a.n.p. and excited by angiotensin II, but four neurones in the p.v.n. were inhibited by a.n.p. and excited by arginine-vasopressin.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe H., Inoue M., Matsuo T., Ogata N. The effects of vasopressin on electrical activity in the guinea-pig supraoptic nucleus in vitro. J Physiol. 1983 Apr;337:665–685. doi: 10.1113/jphysiol.1983.sp014648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes-Rodrigues J., McCann S. M., Rogers L. C., Samson W. K. Atrial natriuretic factor inhibits dehydration- and angiotensin II-induced water intake in the conscious, unrestrained rat. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8720–8723. doi: 10.1073/pnas.82.24.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie M. G., Geller D. M., Cole B. R., Siegel N. R., Fok K. F., Adams S. P., Eubanks S. R., Galluppi G. R., Needleman P. Purification and sequence analysis of bioactive atrial peptides (atriopeptins). Science. 1984 Jan 6;223(4631):67–69. doi: 10.1126/science.6419347. [DOI] [PubMed] [Google Scholar]
- Dudek F. E., Hatton G. I., Macvicar B. A. Intracellular recordings from the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1980 Apr;301:101–114. doi: 10.1113/jphysiol.1980.sp013192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn T. G., de Bold M. L., de Bold A. J. The amino acid sequence of an atrial peptide with potent diuretic and natriuretic properties. Biochem Biophys Res Commun. 1983 Dec 28;117(3):859–865. doi: 10.1016/0006-291x(83)91675-3. [DOI] [PubMed] [Google Scholar]
- Gronan R. J., York D. H. Effects of angiotensin II and acetylcholine on neurons in the preoptic area. Brain Res. 1978 Oct 6;154(1):172–177. doi: 10.1016/0006-8993(78)91067-3. [DOI] [PubMed] [Google Scholar]
- Inenaga K., Yamashita H. Excitation of neurones in the rat paraventricular nucleus in vitro by vasopressin and oxytocin. J Physiol. 1986 Jan;370:165–180. doi: 10.1113/jphysiol.1986.sp015928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobowitz D. M., Skofitsch G., Keiser H. R., Eskay R. L., Zamir N. Evidence for the existence of atrial natriuretic factor-containing neurons in the rat brain. Neuroendocrinology. 1985 Jan;40(1):92–94. doi: 10.1159/000124058. [DOI] [PubMed] [Google Scholar]
- Kannan H., Yamashita H. Connections of neurons in the region of the nucleus tractus solitarius with the hypothalamic paraventricular nucleus: their possible involvement in neural control of the cardiovascular system in rats. Brain Res. 1985 Mar 11;329(1-2):205–212. doi: 10.1016/0006-8993(85)90526-8. [DOI] [PubMed] [Google Scholar]
- Kawata M., Nakao K., Morii N., Kiso Y., Yamashita H., Imura H., Sano Y. Atrial natriuretic polypeptide: topographical distribution in the rat brain by radioimmunoassay and immunohistochemistry. Neuroscience. 1985 Nov;16(3):521–546. doi: 10.1016/0306-4522(85)90190-3. [DOI] [PubMed] [Google Scholar]
- Knowles W. D., Phillips M. I. Angiotensin II responsive cells in the organum vasculosum lamina terminalis (OVLT) recorded in hypothalamic brain slices. Brain Res. 1980 Sep 15;197(1):256–259. doi: 10.1016/0006-8993(80)90455-2. [DOI] [PubMed] [Google Scholar]
- Lengvári I., Liposits Z., Vigh S., Schally A. V., Flerkó B. The origin and ultrastructural characteristics of corticotropin-releasing factor (CRF)-immunoreactive nerve fibers in the posterior pituitary of the rat. Cell Tissue Res. 1985;240(2):467–471. doi: 10.1007/BF00222361. [DOI] [PubMed] [Google Scholar]
- Lind R. W., Swanson L. W., Ganten D. Organization of angiotensin II immunoreactive cells and fibers in the rat central nervous system. An immunohistochemical study. Neuroendocrinology. 1985 Jan;40(1):2–24. doi: 10.1159/000124046. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Katsuura G., Nakao K., Imura H. Antidipsogenic action of alpha-human atrial natriuretic polypeptide administered intracerebroventricularly in rats. Neurosci Lett. 1985 Jul 4;58(1):1–6. doi: 10.1016/0304-3940(85)90319-2. [DOI] [PubMed] [Google Scholar]
- Okuya S., Inenaga K., Kaneko T., Yamashita H. Angiotensin II sensitive neurons in the supraoptic nucleus, subfornical organ and anteroventral third ventricle of rats in vitro. Brain Res. 1987 Jan 27;402(1):58–67. doi: 10.1016/0006-8993(87)91047-x. [DOI] [PubMed] [Google Scholar]
- Pittman Q. J., Hatton J. D., Bloom F. E. Spontaneous activity in perfused hypothalamic slices: dependence on calcium content of perfusate. Exp Brain Res. 1981;42(1):49–52. doi: 10.1007/BF00235728. [DOI] [PubMed] [Google Scholar]
- Samson W. K. Atrial natriuretic factor inhibits dehydration and hemorrhage-induced vasopressin release. Neuroendocrinology. 1985 Mar;40(3):277–279. doi: 10.1159/000124085. [DOI] [PubMed] [Google Scholar]
- Saper C. B., Standaert D. G., Currie M. G., Schwartz D., Geller D. M., Needleman P. Atriopeptin-immunoreactive neurons in the brain: presence in cardiovascular regulatory areas. Science. 1985 Mar 1;227(4690):1047–1049. doi: 10.1126/science.2858127. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Tanaka I., Misono K. S., Inagami T. Atrial natriuretic factor in rat hypothalamus, atria and plasma: determination by specific radioimmunoassay. Biochem Biophys Res Commun. 1984 Oct 30;124(2):663–668. doi: 10.1016/0006-291x(84)91606-1. [DOI] [PubMed] [Google Scholar]
- Yamashita H., Inenaga K., Koizumi K. Possible projections from regions of paraventricular and supraoptic nuclei to the spinal cord: electrophysiological studies. Brain Res. 1984 Apr 2;296(2):373–378. doi: 10.1016/0006-8993(84)90077-5. [DOI] [PubMed] [Google Scholar]


