Abstract
1. Phasic bursting by magnocellular neuroendocrine cells (m.n.c.s) in vivo causes increased vasopressin release from axon terminals in the neurohypophysis. In the supraoptic nucleus of the coronal hypothalamic slice thirty-two of sixty-five m.n.c.s recorded intracellularly displayed repetitive bursting, either spontaneously or during a low level of tonic current injection. 2. Of the thirty-two repetitive bursters, twenty-four received no apparent patterned synaptic input and the phasic burst behaviour was voltage dependent. The evidence for these cells being bursting pace-makers and the underlying mechanism driving bursting were further investigated. 3. Phasic bursting by m.n.c.s is usually contingent upon two depolarizing events: a slow depolarization (s.d.) between bursts that brings the membrane potential to burst threshold, and the spike depolarizing after-potential (d.a.p.). One or several d.a.p.s can initiate a burst by summing to form a plateau potential which sustains firing. 4. Of eight phasic cells exposed to tetrodotoxin (TTX) and tonically depolarized with current injection, two cells retained the phasic burst pattern and underlying plateau potentials. Of the remaining six cells in TTX, three of four cells tested regained phasic firing with plateau potentials following the addition of Sr2+, a Ca2+ agonist. Evoked post-synaptic potentials were demonstrably blocked throughout TTX exposure, firmly establishing that some m.n.c.s are bursting pace-makers. 5. The s.d., d.a.p. and plateau potential were retained in TTX or low-Na+ saline, augmented in Sr2+ and blocked in low-Ca2+ saline. All three events were activated at membrane potentials depolarized from -70 mV but steadily inactivated with increasing hyperpolarization to -90 mV. The s.d. and d.a.p. apparently represented partial activation of the same process that drives a burst, the plateau potential. 6. Hyperpolarizing pulses of constant current revealed an apparent decrease in cell conductance underlying the s.d., d.a.p. and plateau potential which was not due to membrane rectification. The plateau potential was reduced in low Na+ and eliminated in low Ca2+. However, it remained relatively unaffected by altering the external K+ concentration and it did not reverse below -90 mV, suggesting a less important role for K+ movement relative to Ca2+ or Na+. A hyperpolarizing pulse during the s.d., d.a.p. or plateau potential probably momentarily inactivated inward Ca2+ current, causing the apparent conductance decrease.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

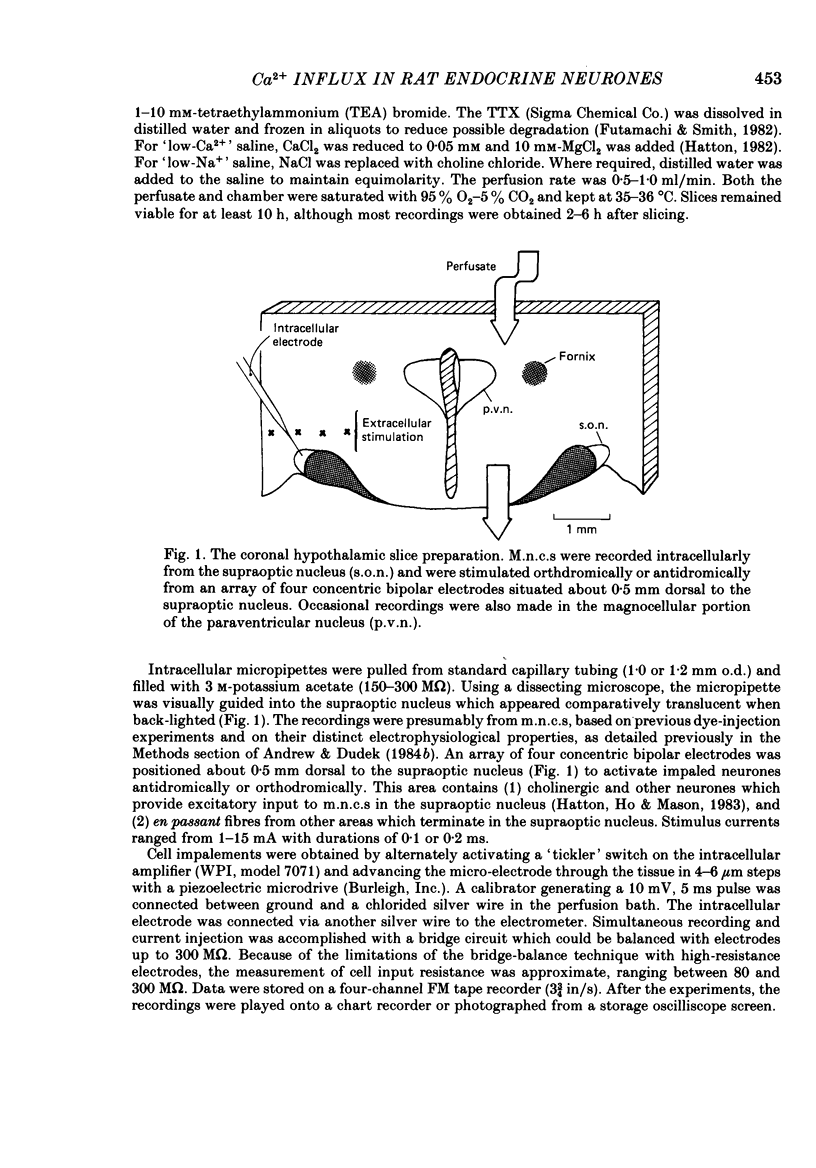
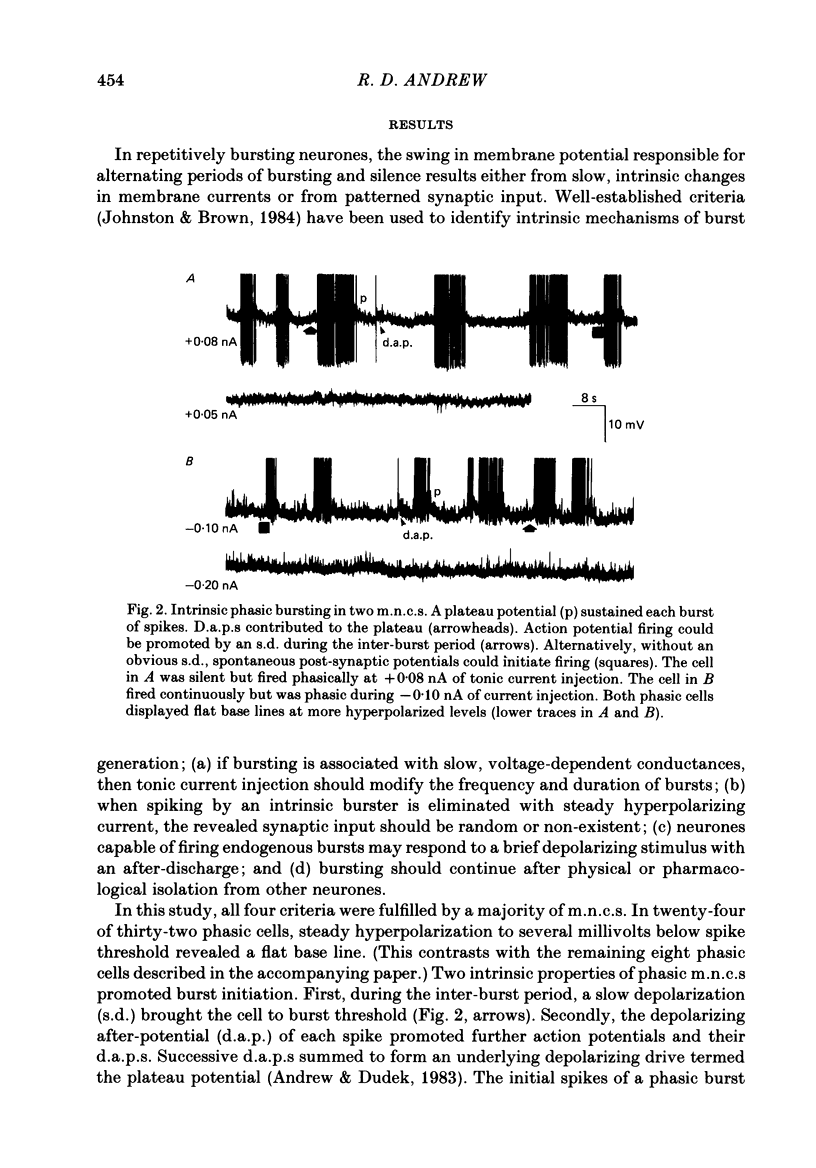
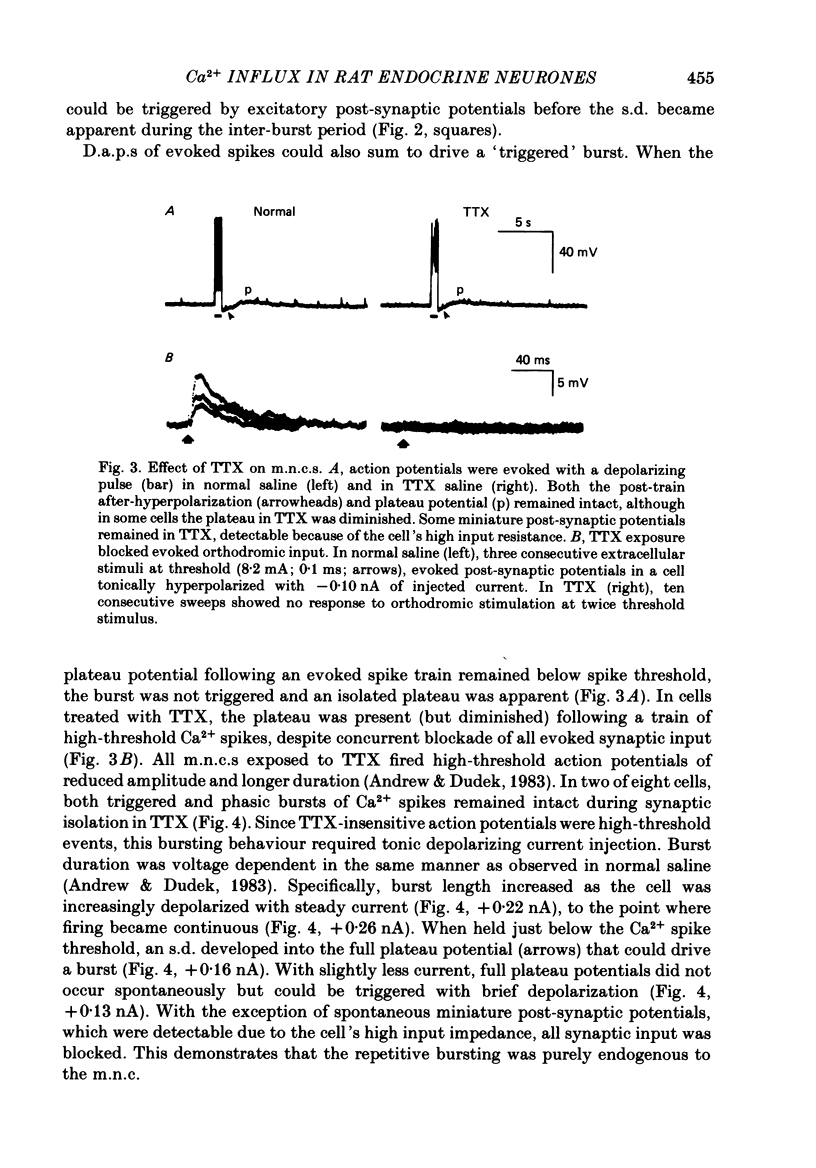
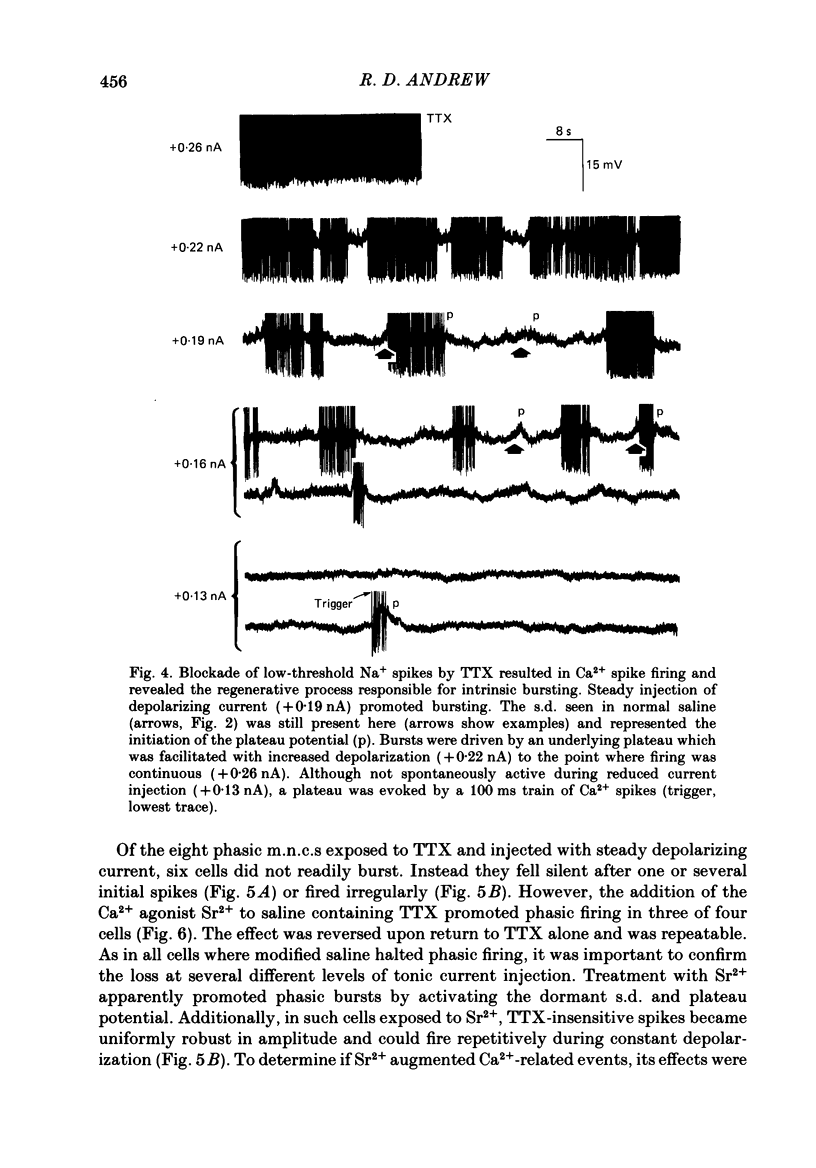
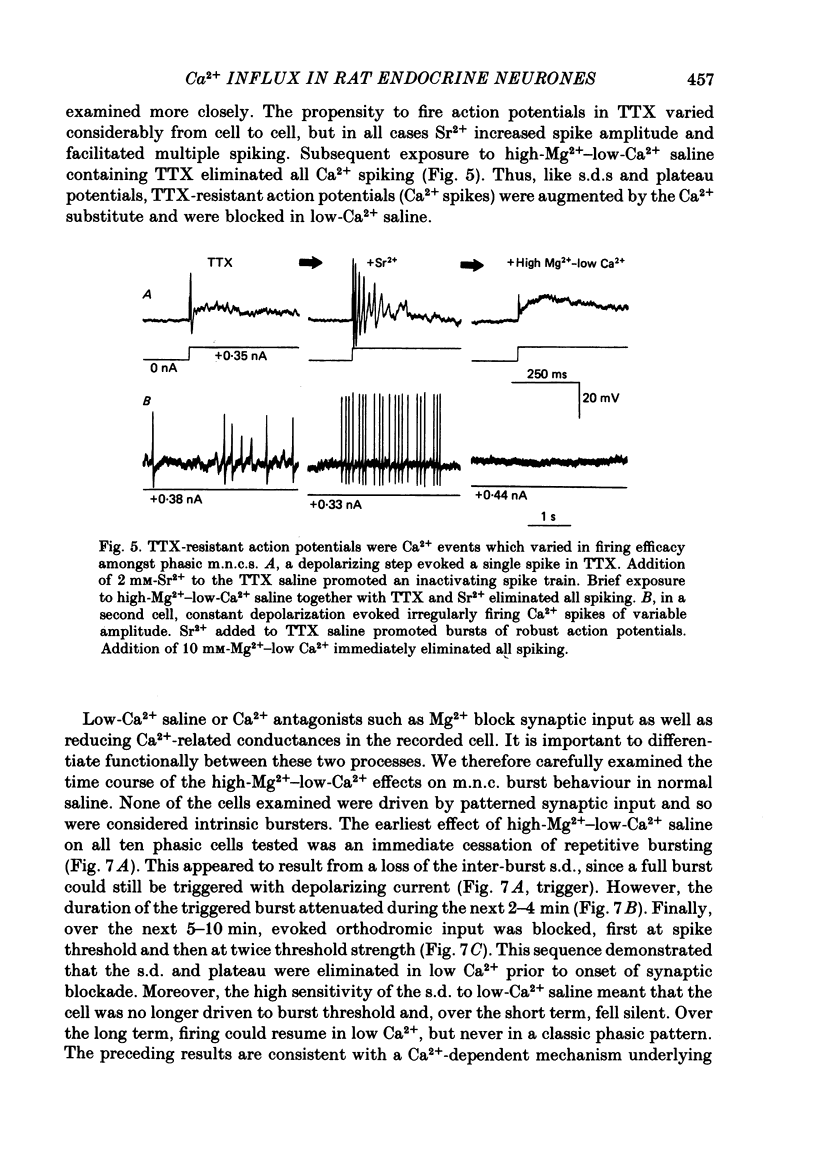





Selected References
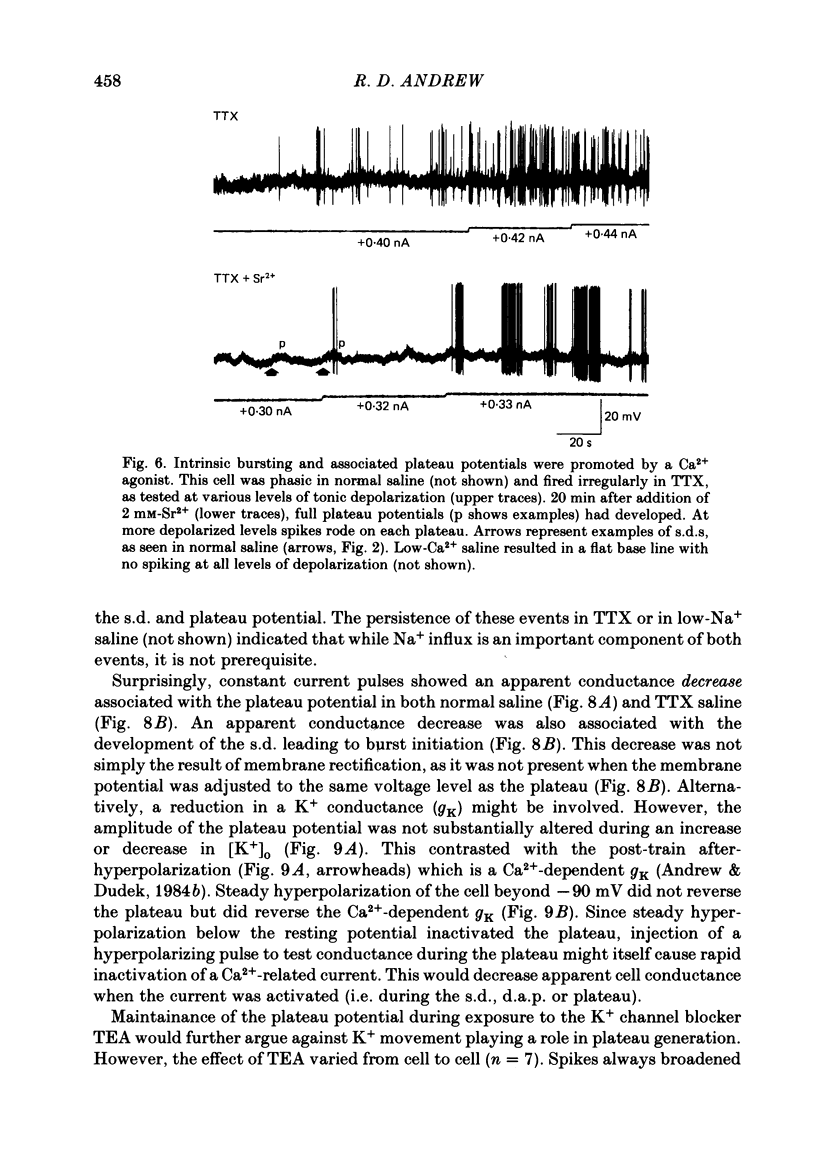
These references are in PubMed. This may not be the complete list of references from this article.
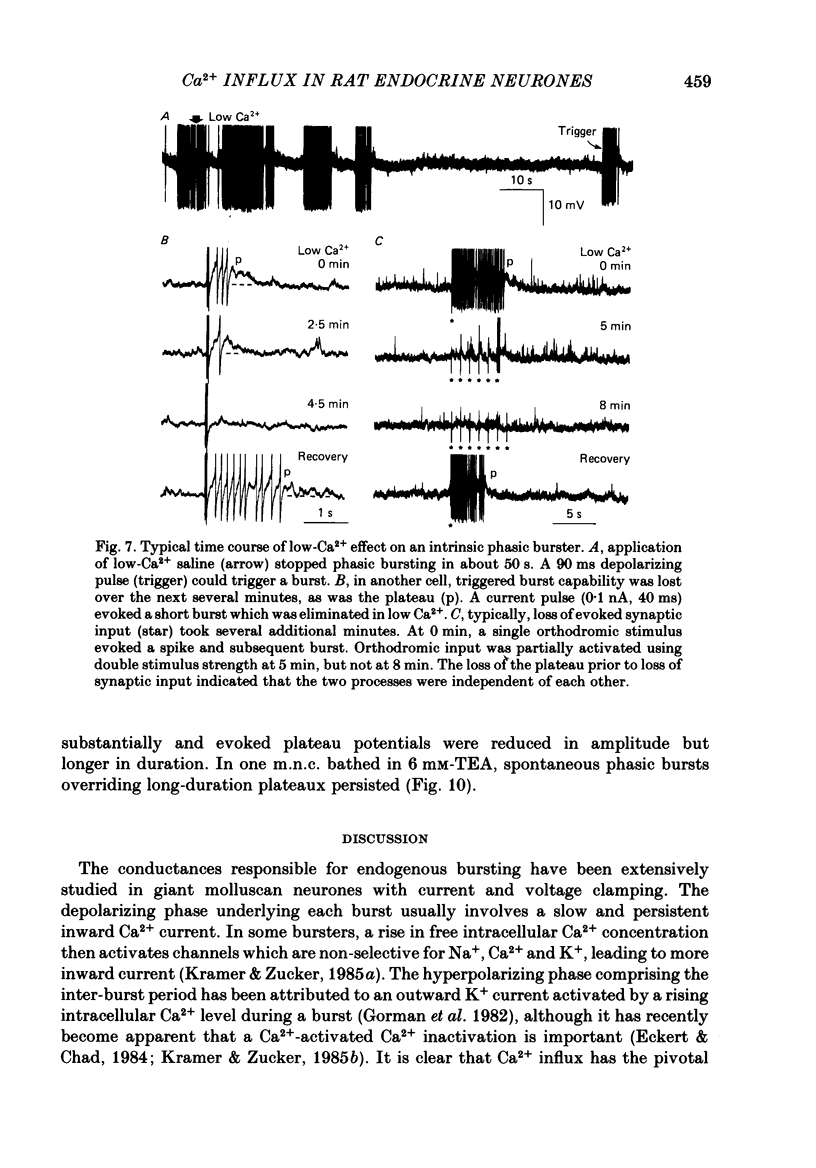
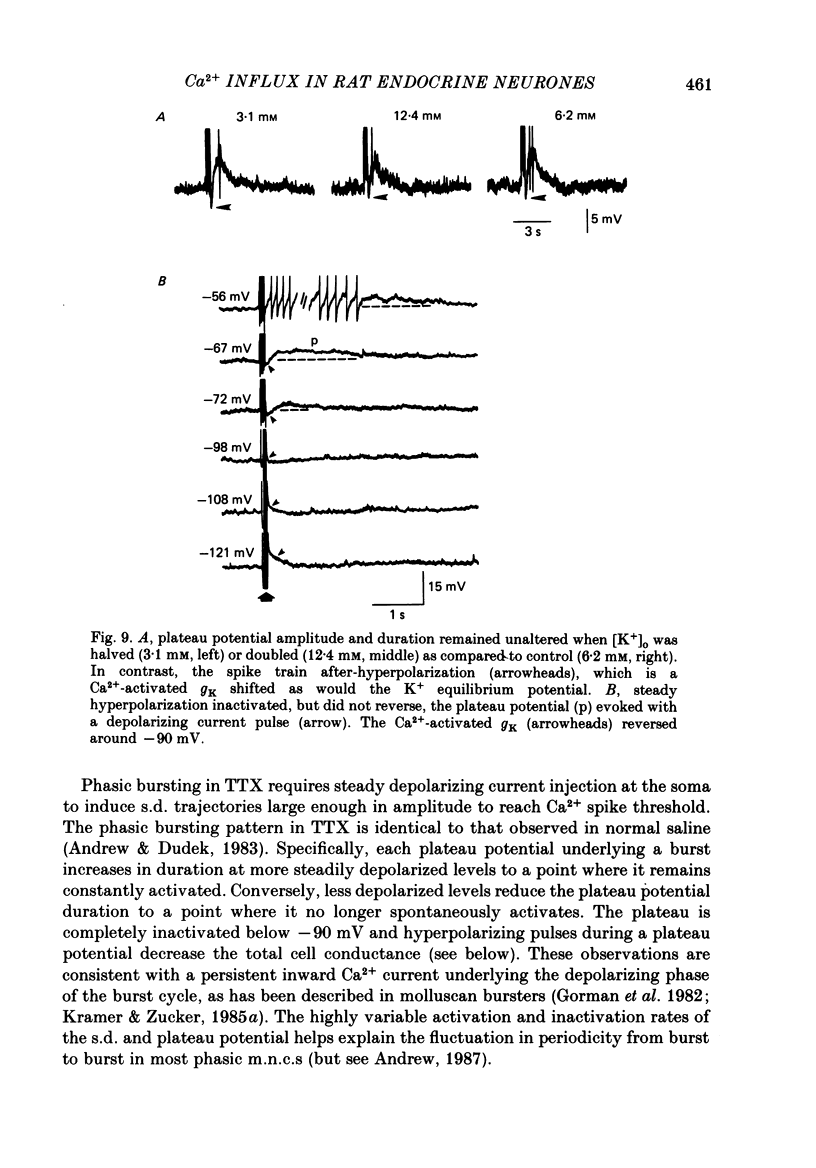
- Alkon D. L., Shoukimas J. J., Heldman E. Calcium-mediated decrease of a voltage-dependent potassium current. Biophys J. 1982 Dec;40(3):245–250. doi: 10.1016/S0006-3495(82)84479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew R. D., Dudek F. E. Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells. J Neurophysiol. 1984 Mar;51(3):552–566. doi: 10.1152/jn.1984.51.3.552. [DOI] [PubMed] [Google Scholar]
- Andrew R. D., Dudek F. E. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983 Sep 9;221(4615):1050–1052. doi: 10.1126/science.6879204. [DOI] [PubMed] [Google Scholar]
- Andrew R. D., Dudek F. E. Intrinsic inhibition in magnocellular neuroendocrine cells of rat hypothalamus. J Physiol. 1984 Aug;353:171–185. doi: 10.1113/jphysiol.1984.sp015330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew R. D. Isoperiodic bursting by magnocellular neuroendocrine cells in the rat hypothalamic slice. J Physiol. 1987 Mar;384:467–477. doi: 10.1113/jphysiol.1987.sp016464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R. J., Leng G. Relative efficiency of neural firing patterns for vasopressin release in vitro. Neuroendocrinology. 1981 Nov;33(5):295–299. doi: 10.1159/000123248. [DOI] [PubMed] [Google Scholar]
- Bourque C. W., Renaud L. P. Activity dependence of action potential duration in rat supraoptic neurosecretory neurones recorded in vitro. J Physiol. 1985 Jun;363:429–439. doi: 10.1113/jphysiol.1985.sp015720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulloch A. G., Willows A. O. Physiological basis of feeding behavior in Tritonia diomedea. III. Role of depolarizing afterpotentials. J Neurobiol. 1981 Sep;12(5):515–532. doi: 10.1002/neu.480120510. [DOI] [PubMed] [Google Scholar]
- Dekin M. S., Richerson G. B., Getting P. A. Thyrotropin-releasing hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science. 1985 Jul 5;229(4708):67–69. doi: 10.1126/science.3925552. [DOI] [PubMed] [Google Scholar]
- Dutton A., Dyball R. E. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979 May;290(2):433–440. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Chad J. E. Inactivation of Ca channels. Prog Biophys Mol Biol. 1984;44(3):215–267. doi: 10.1016/0079-6107(84)90009-9. [DOI] [PubMed] [Google Scholar]
- Eckert R., Lux H. D. Calcium-dependent depression of a late outward current in snail neurons. Science. 1977 Jul 29;197(4302):472–475. doi: 10.1126/science.17921. [DOI] [PubMed] [Google Scholar]
- Futamachi K., Smith T. G., Jr Action of tetrodotoxin on pacemaker conductances in Aplysia neurons. Brain Res. 1982 Feb 11;233(2):424–430. doi: 10.1016/0006-8993(82)91218-5. [DOI] [PubMed] [Google Scholar]
- Gorman A. L., Hermann A., Thomas M. V. Ionic requirements for membrane oscillations and their dependence on the calcium concentration in a molluscan pace-maker neurone. J Physiol. 1982 Jun;327:185–217. doi: 10.1113/jphysiol.1982.sp014227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gähwiler B. H., Dreifuss J. J. Phasically firing neurons in long-term cultures of the rat hypothalamic supraoptic area: pacemaker and follower cells. Brain Res. 1979 Nov 9;177(1):95–103. doi: 10.1016/0006-8993(79)90920-x. [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Ho Y. W., Mason W. T. Synaptic activation of phasic bursting in rat supraoptic nucleus neurones recorded in hypothalamic slices. J Physiol. 1983 Dec;345:297–317. doi: 10.1113/jphysiol.1983.sp014979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton G. I. Phasic bursting activity of rat paraventricular neurones in the absence of synaptic transmission. J Physiol. 1982 Jun;327:273–284. doi: 10.1113/jphysiol.1982.sp014231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D., Brown T. H. The synaptic nature of the paroxysmal depolarizing shift in hippocampal neurons. Ann Neurol. 1984;16 (Suppl):S65–S71. doi: 10.1002/ana.410160711. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Zucker R. S. Calcium-dependent inward current in Aplysia bursting pace-maker neurones. J Physiol. 1985 May;362:107–130. doi: 10.1113/jphysiol.1985.sp015666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. H., Zucker R. S. Calcium-induced inactivation of calcium current causes the inter-burst hyperpolarization of Aplysia bursting neurones. J Physiol. 1985 May;362:131–160. doi: 10.1113/jphysiol.1985.sp015667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P., Cooke I. M., Vincent J. D. Regenerative responses of long duration recorded intracellularly from dispersed cell cultures of fetal mouse hypothalamus. J Neurophysiol. 1982 Nov;48(5):1121–1141. doi: 10.1152/jn.1982.48.5.1121. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol. 1985 Jul;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. Ionic mechanisms of rhythmic firing. Symp Soc Exp Biol. 1983;37:1–28. [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Schwindt P., Crill W. Role of a persistent inward current in motoneuron bursting during spinal seizures. J Neurophysiol. 1980 May;43(5):1296–1318. doi: 10.1152/jn.1980.43.5.1296. [DOI] [PubMed] [Google Scholar]
- Sigvardt K. A., Grillner S., Wallén P., Van Dongen P. A. Activation of NMDA receptors elicits fictive locomotion and bistable membrane properties in the lamprey spinal cord. Brain Res. 1985 Jun 17;336(2):390–395. doi: 10.1016/0006-8993(85)90676-6. [DOI] [PubMed] [Google Scholar]
- Thompson S. H., Smith S. J. Depolarizing afterpotentials and burst production in molluscan pacemaker neurons. J Neurophysiol. 1976 Jan;39(1):153–161. doi: 10.1152/jn.1976.39.1.153. [DOI] [PubMed] [Google Scholar]
- Tokimasa T. Intracellular Ca2+-ions inactivate K+-current in bullfrog sympathetic neurons. Brain Res. 1985 Jul 1;337(2):386–391. doi: 10.1016/0006-8993(85)90081-2. [DOI] [PubMed] [Google Scholar]


