Abstract
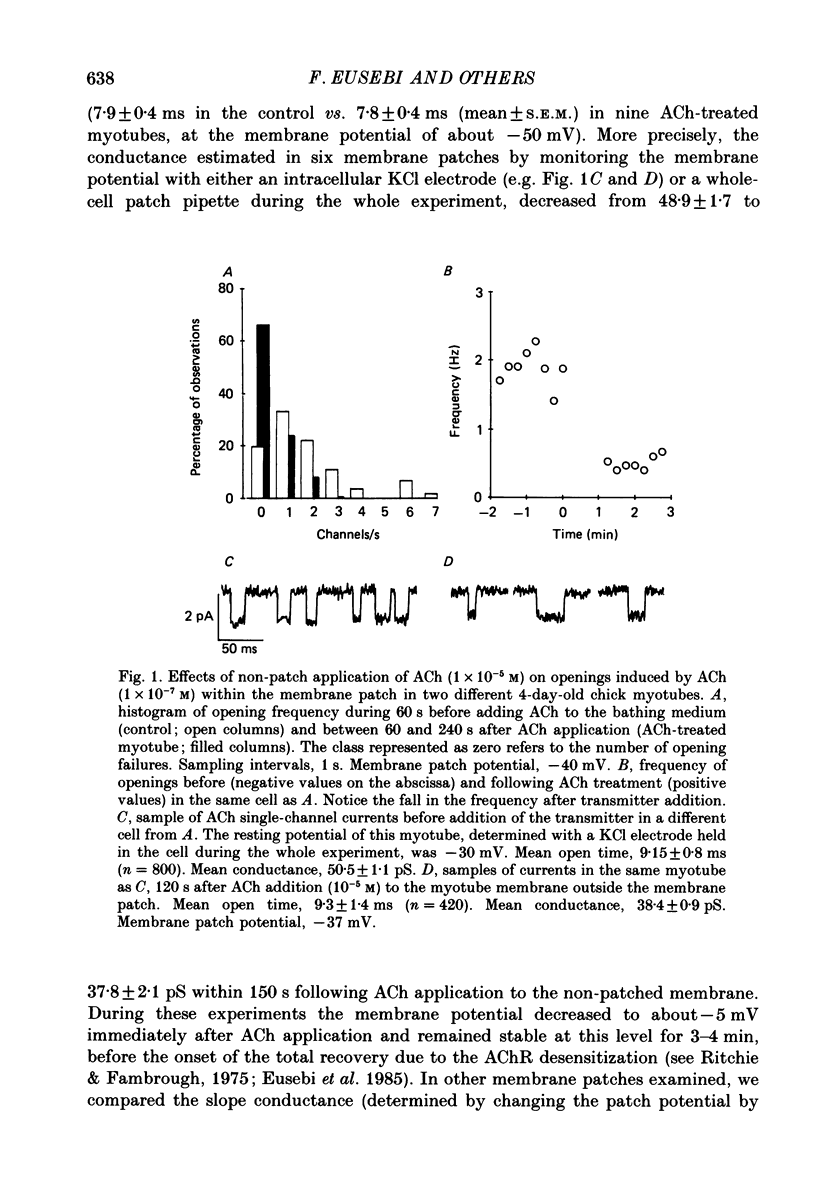
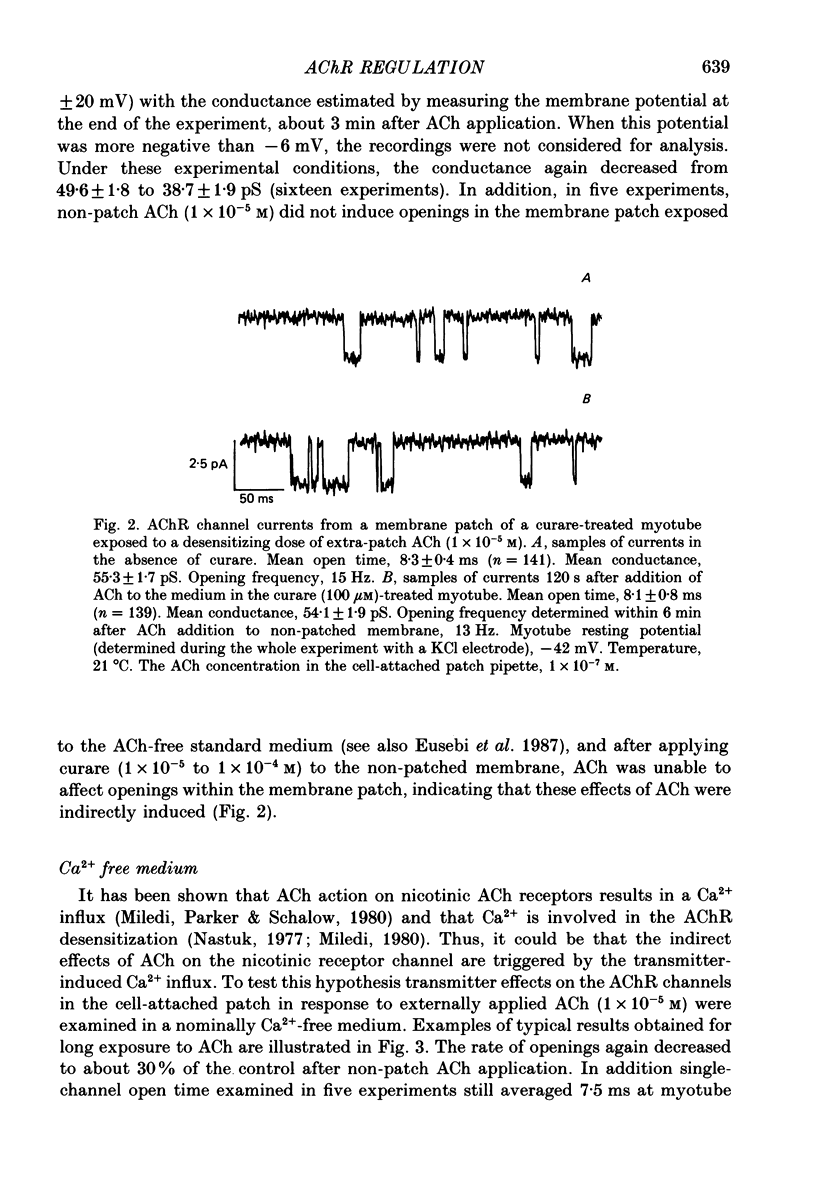
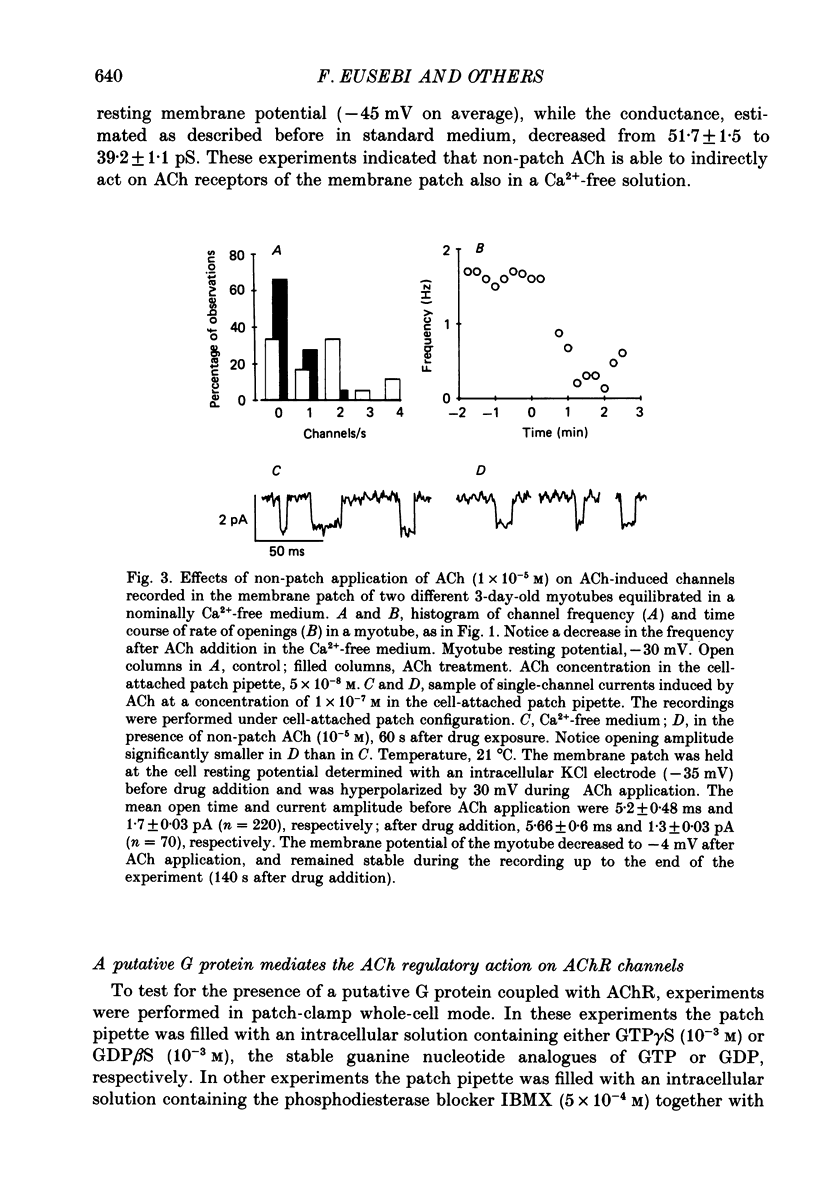
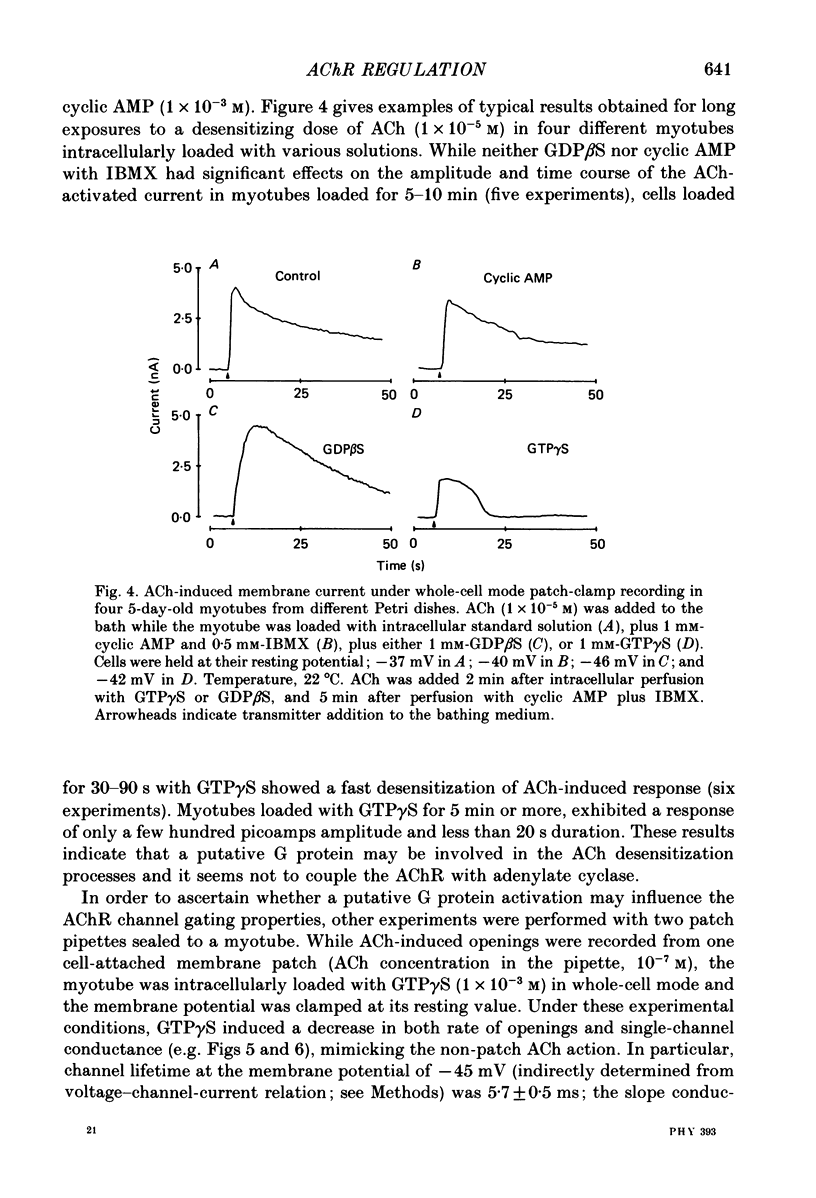
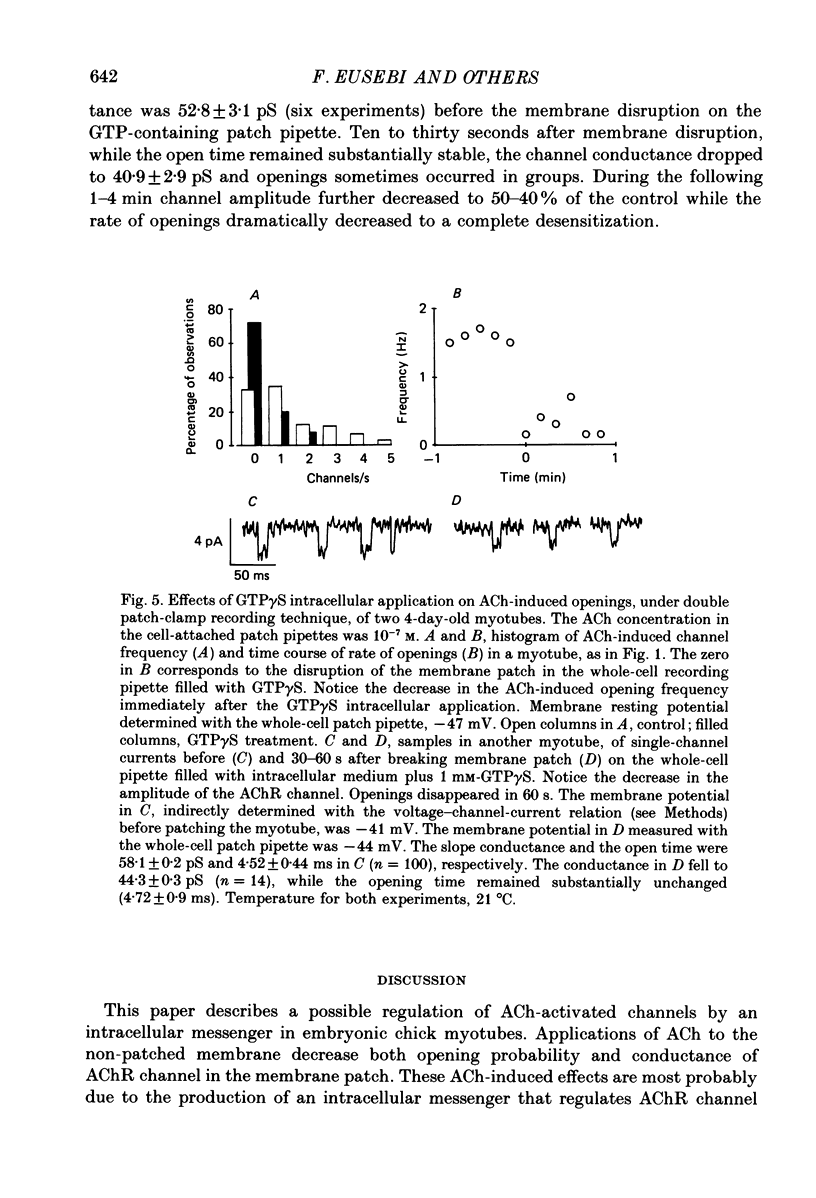
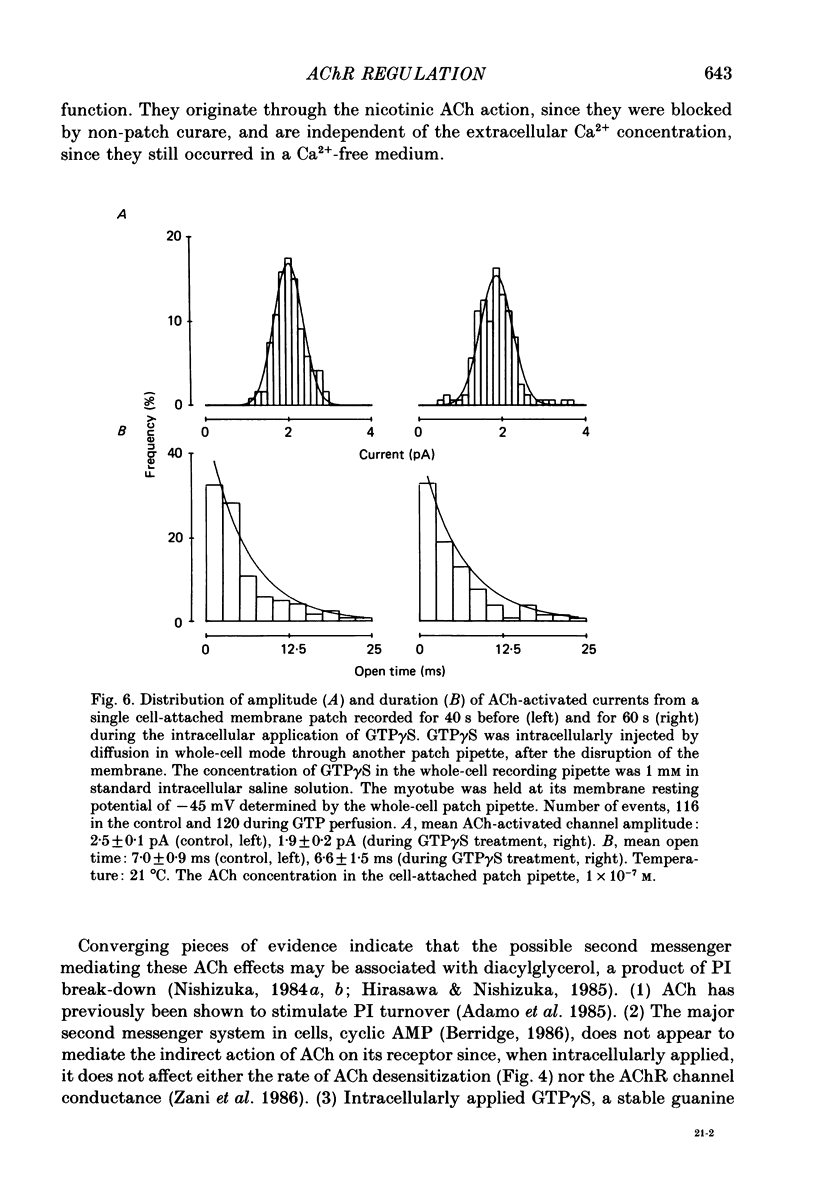
1. Single-channel currents induced by acetylcholine (ACh) were recorded from unstriated and non-innervated embryonic chick myotubes using the cell-attached patch-clamp technique. 2. ACh applied to the non-patched membrane decreased both channel opening probability and conductance. These ACh-induced effects occurred also when the non-patched membrane was exposed to nominally Ca2+-free extracellular medium, but were absent when it was treated with curare. 3. ACh-induced membrane current recorded under whole-cell patch-clamp conditions decreased in amplitude and time course when myotubes were intracellularly loaded with guanosine-5'-O-(3-thiotriphosphate) GTP gamma S), but not with guanosine-5'-O-(2-thiodiphosphate) (GDP beta S) or cyclic adenosine-5'-monophosphate (cyclic AMP). Internal perfusion of GTP gamma S affected the ACh-induced openings in a similar manner to the non-patch ACh application. 4. These results suggest that ACh, in addition to its direct effect, acts indirectly on the nicotinic receptor channels by delivering an intracellular messenger and through the activation of a putative G protein.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamo S., Zani B. M., Nervi C., Senni M. I., Molinaro M., Eusebi F. Acetylcholine stimulates phosphatidylinositol turnover at nicotinic receptors of cultured myotubes. FEBS Lett. 1985 Oct 7;190(1):161–164. doi: 10.1016/0014-5793(85)80449-x. [DOI] [PubMed] [Google Scholar]
- Adamo S., Zani B., Siracusa G., Molinaro M. Expression of differentiative traits in the absence of cell fusion during myogenesis in culture. Cell Differ. 1976 Apr;5(1):53–67. doi: 10.1016/0045-6039(76)90015-4. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Deshpande S. S., Aracava Y., Alkondon M., Daly J. W. A possible involvement of cyclic AMP in the expression of desensitization of the nicotinic acetylcholine receptor. A study with forskolin and its analogs. FEBS Lett. 1986 Apr 7;199(1):113–120. doi: 10.1016/0014-5793(86)81235-2. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Cell signalling through phospholipid metabolism. J Cell Sci Suppl. 1986;4:137–153. doi: 10.1242/jcs.1986.supplement_4.9. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M. D., Huganir R., Greengard P. Protein phosphorylation and neuronal function. J Neurochem. 1985 Jul;45(1):11–23. doi: 10.1111/j.1471-4159.1985.tb05468.x. [DOI] [PubMed] [Google Scholar]
- Eusebi F., Molinaro M., Zani B. M. Agents that activate protein kinase C reduce acetylcholine sensitivity in cultured myotubes. J Cell Biol. 1985 Apr;100(4):1339–1342. doi: 10.1083/jcb.100.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Sakmann B. Multiple conductance states of single acetylcholine receptor channels in embryonic muscle cells. Nature. 1981 Dec 3;294(5840):462–464. doi: 10.1038/294462a0. [DOI] [PubMed] [Google Scholar]
- Hirasawa K., Nishizuka Y. Phosphatidylinositol turnover in receptor mechanism and signal transduction. Annu Rev Pharmacol Toxicol. 1985;25:147–170. doi: 10.1146/annurev.pa.25.040185.001051. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Delcour A. H., Greengard P., Hess G. P. Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature. 1986 Jun 19;321(6072):774–776. doi: 10.1038/321774a0. [DOI] [PubMed] [Google Scholar]
- Michell B., Kirk C. G-protein control of inositol phosphate hydrolysis. Nature. 1986 Sep 11;323(6084):112–113. doi: 10.1038/323112b0. [DOI] [PubMed] [Google Scholar]
- Middleton P., Jaramillo F., Schuetze S. M. Forskolin increases the rate of acetylcholine receptor desensitization at rat soleus endplates. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4967–4971. doi: 10.1073/pnas.83.13.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Intracellular calcium and desensitization of acetylcholine receptors. Proc R Soc Lond B Biol Sci. 1980 Sep 26;209(1176):447–452. doi: 10.1098/rspb.1980.0106. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Transmitter induced calcium entry across the post-synaptic membrane at frog end-plates measured using arsenazo III. J Physiol. 1980 Mar;300:197–212. doi: 10.1113/jphysiol.1980.sp013158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E. J., Walaas S. I., Greengard P. Neuronal phosphoproteins: physiological and clinical implications. Science. 1984 Sep 21;225(4668):1357–1364. doi: 10.1126/science.6474180. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Zani B. M., Grassi F., Molinaro M., Monaco L., Eusebi F. Cyclic AMP regulates the life time of acetylcholine-activated channels in cultured myotubes. Biochem Biophys Res Commun. 1986 Oct 15;140(1):243–249. doi: 10.1016/0006-291x(86)91082-x. [DOI] [PubMed] [Google Scholar]


