Abstract
This paper provides an overview of the functional anatomy of the structures responsible for controlling urinary continence under stress. The stress continence control system can be divided into two parts: the system responsible for bladder neck support, and the system responsible for sphincteric closure. Age- and injury-related changes in each of these systems are discussed. Understanding the pathophysiology of incontinence on the anatomical level will help to lead to identification of specific defects, thereby allowing better individualized treatment for the incontinent patient.
Keywords: pelvic floor, anatomy, stress urinary incontinence, aging, injury
Urinary incontinence is a common condition in women. The overall prevalence ranges from 8.5% to 38% depending upon age, parity and definition (1, 2). The majority of women with incontinence have stress incontinence (3), which is treated initially using conservative therapy, and thereafter by surgery. Despite the extent of this problem there have been few advances in the treatment of this disorder. Although many surgical procedures exist for stress incontinence, the majority have in common the major principle of improving bladder neck support (4, 5).
Understanding how the structure and function of the pelvic floor provide bladder neck support helps guide treatment selection and effect. Knowing the effect that the ability to perform a pelvic muscle contraction might have on the treatment of incontinence is important. One example: pelvic muscle exercises as a treatment for incontinence would only be an option for those patients with pelvic muscles having sufficient residual innervation to control those muscles. This paper is intended to review the anatomy and function of the different aspects of the pelvic floor and stress continence control system.
THE STRESS CONTINENCE CONTROL SYSTEM
We have examined the relative contributions of the passive and active systems acting to close the urethra using urodynamic analyses (6). Although such analyses provide useful theoretical insights, they provide few insights on structure–function relationships because they fail to identify the anatomic basis for the active or passive contributions to urethral closure. The stress continence control system can be divided anatomically into two parts: the urethral support system and the sphincteric closure system.
THE URETHRAL SUPPORT SYSTEM
The urethral support system consists of all the structures extrinsic to the urethra that provide a supportive layer upon which the urethra rests (7). The major components of this supportive structure include the anterior vagina, the endopelvic fascia, the arcus tendineus fasciae pelvis, and the levator ani muscles (Fig. 1).
Fig. 1.
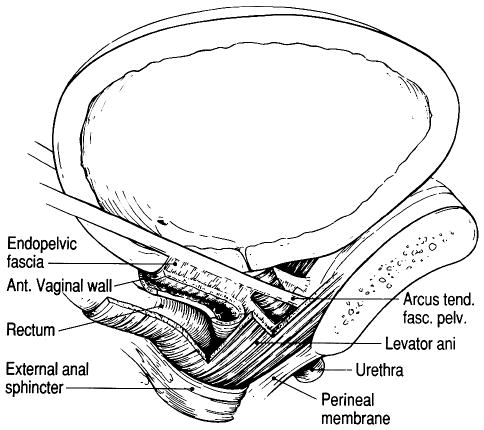
Lateral view shows the components of the urethral support system. Note how the levator ani muscles support the rectum, vagina, and urethrovesical neck. Also note how the endopelvic fascia beside the urethra attaches to the levator ani muscle. A contraction of the muscle would lead to elevation of the urethrovesical neck.
The endopelvic fascia is a dense fibrous connective tissue layer, which surrounds the vagina and attaches it to the arcus tendineus fasciae pelvis laterally. The arcus tendineus fasciae pelvis in turn is attached to the pubic bone ventrally and to the ischial spine dorsally. The arcus tendineus fasciae pelvis is a tensile structure located bilaterally on either side of the urethra and vagina. It acts like the catenary-shaped cable of a suspension bridge and provides the support needed to suspend the urethra on the anterior vaginal wall. Although well-defined near its origin as a fibrous band at the pubic bone, the arcus tendineus fasciae pelvis becomes a broad aponeurotic structure as it passes dorsally to the ischial spine. It therefore appears as a sheet of fascia as it fuses with the endopelvic fascia, where it merges with the levator ani muscles (Fig. 2) (7).
Fig. 2.
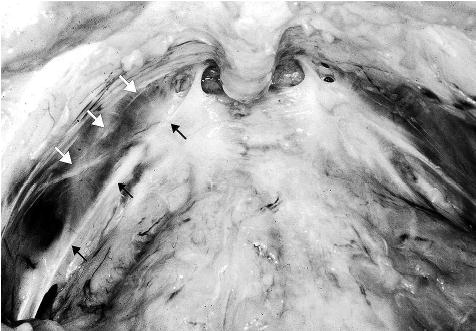
Photo of the space of Retzius. White arrows shows the arcus tendineus levator ani and the black arrows the arcus tendineus fasciae pelvis.
The levator ani muscle consists of three parts: the pubococcygeus, the puborectalis, and the iliococcygeus muscles. The pubococcygeus and the puborectalis muscles form a U-shape as they originate from the pubic bone on either side of the midline and pass behind the rectum to form a sling. This sling of muscle is composed of predominantly Type I striated muscle fibers and therefore is suited to maintaining constant tone (8). It is this constant tone that normally keeps the urogenital hiatus (9) closed. The iliococcygeus muscle arises laterally from the arcus tendineus levator ani and forms a horizontal sheet that spans the opening in the posterior region of the pelvis, thereby providing a “shelf” upon which the pelvic organs rest.
Functionally, the levator ani muscle and the endopelvic fascia serve an interactive role in maintaining continence and pelvic support. In a hard cough intraabdominal pressure can increase suddenly by about 150 cm H2O and ultrasound studies have shown that this causes the proximal urethra to undergo a midsagittal plane caudodorsal displacement of about 10 mm (10). This displacement is evidence that the inferior abdominal contents are forced to move caudodorsally (“downwards”) during a cough, presumably due to a simultaneous contraction of the diaphragm and abdominal wall muscles. If the diaphragm contracts and shortens then, because the abdominal contents are essentially incompressible, either the pelvic floor and/or the abdominal wall must stretch. The downward motion of the bladder neck visible in the ultrasound picture means that its surrounding tissues acquire downward momentum (recall from high school physics that momentum is defined as the product of a mass times its velocity). This downward momentum must then be arrested by stretch resistance of the pelvic floor structures. As the downward momentum of the abdominal contents is slowed by the stretch of the pelvic floor structures, this fluid movement compresses the proximal intraabdominal portion of the urethra against the underlying supportive layer, which is composed of the endopelvic fasciae, the vagina, and the levator ani muscles. We can estimate the stretch-resistance of the supportive layer to the dorsocaudal displacement, a resistance that is known to bioengineers as “stiffness”. If we divide the 150 cm H2O cough-related increase in intraabdominal pressure by the displacement of the bladder neck, the resulting ratio is 150 cm H2O divided by 10 mm, or 15 cm H2O/mm. This stiffness of the pelvic floor means that for about every 15 cm H2O increase in intraabdominal pressure we would expect the healthy pelvic floor to stretch downward 1 mm. The abdominal pressure acts transversely across the urethra, altering the stresses in the wall of the urethra so that its anterior wall is deformed toward its posterior wall, thereby helping to close the urethral lumen and prevent leakage caused by the increase in intravesical pressure.
If there are breaks in the continuity of the endopelvic fascia (11), or if the levator ani muscle were to be damaged (see below), the supportive layer under the urethra would likely be less stiff. In fact, Howard et al. (10) have shown that the stiffness of the bladder neck support is significantly less than in women with stress incontinence than in matched controls. The supportive layer would then provide less resistance to deformation during increases in abdominal pressure and thus closure of the urethral lumen is not ensured, raising the possibility of stress incontinence. An analogy that we have used previously is attempting to halt the flow of water through a garden hose by stepping on it (12). Let us take the same analogy, but refine it slightly. If the hose were lying on a very stiff trampoline, stepping on it would change the stress in the wall of hose wall, leading to a deformation and flattening of the hose cross-sectional area, resulting in closure of the lumen and cessation of water flow, with little indentation or deflection of the trampoline. If, instead, the hose were resting on low-stiffness (or compliant) trampoline, stepping on the hose would tend to cause it to indent the trampoline under it, then the hose and the trampoline would move downward together as the trampoline stretches. While the hose and trampoline move downward together, water could flow almost unabated in the hose. After a delay, the stretch of the trampoline will finally slow and halt the downward movement of the foot and hose, and flow may or may not be stopped. So, a loss in stiffness of the supporting tissues could well alter, and delay, the effect of abdominal pressure on the transverse closure of the urethral lumen.
Additionally, the constant tone maintained by the pelvic muscles relieves the tension placed upon the endopelvic fascia. If the nerves to the levator ani muscle are damaged (such as can occur during childbirth), the denervated muscles would undergo atrophy thereby leaving the responsibility of pelvic organ support upon the endopelvic fascia alone. Over time, these ligaments would exhibit viscoelastic behavior, gradually stretching under the constant load, leading to the development of prolapse.
There are several direct clinical applications for this information. The first concerns the types of damage that can occur to the urethral support system. An example is the paravaginal defect that causes separation in the endopelvic fascia connecting the vagina to the pelvic sidewall. This separation reduces the stiffness of the fascial layer supporting the urethra. When this occurs, increases in abdominal pressure can no longer effectively compress the urethra against the supporting endopelvic fascia in order to close it during increases in abdominal pressure. This paravaginal defect, when present, can be repaired surgically, restoring normal anatomy.
Normal function of the urethral support system requires the contraction of the levator ani muscle, which supports the urethra through the endopelvic fascia. During a cough, there is a simultaneous contraction of the levator ani muscle with the diaphragm and abdominal wall muscles to build abdominal pressure. This levator ani contraction helps to tense the suburethral fascial layer and thereby enhance urethral compression. It also protects the connective tissue from undue stresses. The strength of the levator ani muscle has recently been quantified under isometric conditions (13), and racial differences have also been found in their contractile properties (14).
Elderly striated muscle takes 35% longer to develop the same force as in the young adult, and its maximum force is also diminished by about 35% (15). These changes are not due to alterations in neural recruitment, but due to age-related changes in striated muscle contractility (16). Furthermore, if the striated muscle of the levator ani becomes damaged, or if its innervation is impaired, the muscle contraction will take even longer to develop the same force. Indeed, it may never develop the same force. In either case this leads to a delay or diminution in its mechanical action. In addition, the maximum force that the damaged muscle can produce is likely decreased. This decrease in muscle strength, in turn, will be associated with a loss of pelvic muscle stiffness or resistance to stretch, because striated muscle strength and stiffness are linearly correlated (17). Alternatively, the connection between the muscle and the fascia may be broken (18), and the normal function of the levator ani during a cough is lost. This has important implications for clinical management. Recent evidence from magnetic resonance scans, reviewed in a blinded manner, shows that the connection between the levator ani and the fascia can be seen to be damaged unilaterally or bilaterally in certain patients (19).
Pelvic muscle exercise has been shown to be effective in alleviating stress incontinence in many, but not all, women. If the muscle is normally innervated and is sufficiently attached to the endopelvic fascia, then many women can simply learn to use a voluntary muscle contraction during a cough to help prevent urine loss. This involves proper muscle connection and appropriate contraction timing. If the muscle is denervated as a result of substantial nerve injury, then it may not be possible to rehabilitate the muscle sufficiently to make pelvic muscle exercise an effective strategy. In addition, if the muscle is completely detached from the fascial tissues, then it may be able to contract; but that contraction may not be effective in elevating the urethra or stabilizing its position.
It seems prudent, therefore, before embarking upon pelvic muscle exercise as a therapy to ensure that a woman is capable of proper pelvic muscle contraction. Having a patient cough with a full bladder and measuring the amount of urine lost is quite simple (20). If, by contracting the pelvic muscles prior to and during a cough, a woman is able to decrease her leakage (21), then simply learning to use pelvic muscles may be an effective therapy for her.
THE SPHINCTERIC CLOSURE SYSTEM
The sphincteric closure of the urethra is normally provided by the urethral striated muscles, the urethral smooth muscle, and the vascular elements within the submucosa. (22) (Fig. 3). Each is thought to contribute equally to the resting urethral closure pressure (23).
Fig. 3.
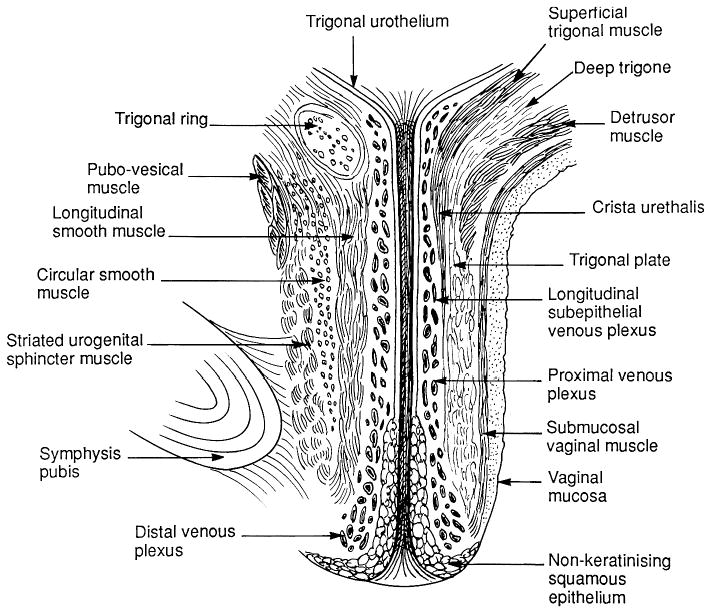
Anatomy of the urethra shown in longitudinal section.
Anatomically the urethra can be divided into percentiles with the internal urethral meatus representing point 0 and the external meatus representing the 100th percentile mark (Table I). The urethra passes through the wall of the bladder at the level of the vesical neck where the detrusor muscle fibers extend below the internal urethra meatus to as far as the 15th percentile. The striated urethral sphincter muscle begins at the termination of the detrusor fibers and extends to the 64th percentile. It is circularly oriented and completely surrounds the smooth muscle of the urethral wall. Starting at the 54th percentile the striated muscles of the urogenital diaphragm, the compressor urethrae and the urethrovaginal sphincter can be seen. They are continuous with the striated urethral sphincter and extend to the 76th percentile. Their fiber direction is no longer circular. The compressor urethrae passes over the urethra to insert into the urogenital diaphragm near the pubic ramus. The urethrovaginal sphincter surrounds both the urethra and the vagina (Fig. 4). The distal terminus of the urethra runs adjacent to, but does not connect with, the bulbocavernosus muscles (24).
Table I.
Urethral topography and urethral and paraurethral structures
| Location (percentile of urethral length) | Region of urethra | Structures |
|---|---|---|
| 0–20th % | Intramural | •Internal urethral meatus |
| •Detrusor loop | ||
| 20–60th % | Midurethra | •Striated urethra sphincter muscle |
| •Smooth muscle | ||
| 60–80th % | Urogenital diaphragm | •Compressor urethrae muscle |
| •Urethrovaginal sphincter | ||
| 80–100th % | Distal urethra | •Smooth muscle |
| •Bulbocavernosus muscle |
Fig. 4.
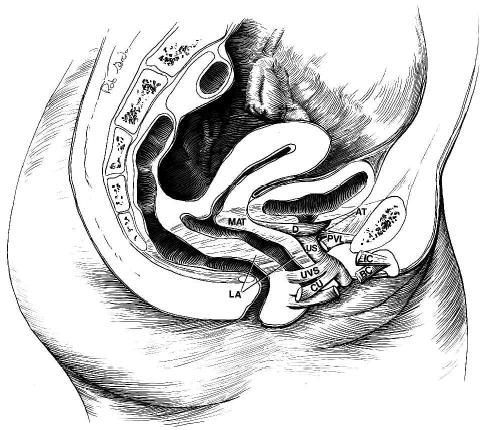
Components of the stress continence control system. VLA = vaginolevator attachment; LA = levator ani muscles; D = detrusor muscle; US = urethral sphincter; CU = compressor urethrae; UVS = urethrovaginal sphincter; AT = arcus tendineus fasciae pelvis; PUL = pubourethral ligament; IC = ischiocavernosus muscle; and BC = bulbocavernosus muscle.
Functionally, the urethral muscles maintain continence in various ways. The U-shaped loop of the detrusor smooth muscle surrounds the proximal urethra favoring its closure by constricting the lumen. The striated urethra sphincter is composed mainly of Type 1 (slow twitch) fibers, which are well-suited to maintain constant tone as well as allow voluntary increases in tone to provide additional continence protection (25). Distally the recruitment of the striated muscle of the urethrovaginal sphincter and the compressor urethrae compress the lumen.
The smooth muscle of the urethra may also play a role in determining stress continence. The lumen is surrounded by a prominent vascular plexus that is thought to contribute to continence by forming a watertight seal via coaptation of the mucosal surfaces. Surrounding this plexus is the inner longitudinal smooth muscle layer, which is in turn surrounded by a circular layer that itself lies inside the outer layer of striated muscle. The smooth muscle layers are present throughout the upper four-fifths of the urethra. The circular configuration of the circular smooth muscle layer and outer striated muscle layer suggests a role in constricting the lumen when these layers contract. The mechanical role of the inner longitudinal smooth muscle layer is presently unresolved. It is possible that contraction of this longitudinal layer may help to open the lumen to initiate micturition, rather than constrict the lumen.
There are several important clinical correlates of urethral muscular anatomy. Perhaps the most important of these is the realization that stress incontinence is caused by problems with the urethral sphincter mechanism as well as with urethral support. Although this is a relatively new concept, the scientific evidence to support this is strong. The usual argument for urethral support playing an important role in stress incontinence is the fact that urethral support operations cure stress incontinence without changing urethral function. Unfortunately this logic is just as flawed as suggesting that obesity is caused by an enlarged stomach because gastric stapling surgery, which makes the stomach smaller, is effective in alleviating obesity. The fact that urethral support operations cure stress incontinence does not implicate urethral hypermobility as the cause of stress incontinence.
Most studies have shown that there is not only a substantial difference in resting urethral closure pressures in normal women and those with stress incontinence, but also the severity of stress incontinence correlates quite well with resting urethral closure pressure.
This loss of urethral closure pressure probably results from age-related deterioration of the urethral musculature as well as neurologic injury (26–29). Substantial decreases in closure pressure are seen with age (23). The ability of exercise to compensate for this is limited. Healthy striated muscle can only, even with the most vigorous exercise, increase its strength by about 30% after an intensive 8–12 week exercise intervention (30). Imagine a woman whose urethral closure pressure was 100 cm H2O as a young woman, but is now 30 cm H2O as an older woman. Let us assume that she successfully increases her urethral striated muscle strength by 30% through an exercise intervention. If there was a one-to-one correspondence between urethral muscle strength and resting closure pressure, she could only increase her resting closure pressure by 30% from 30 to 39 cm H2O, an increment less than one-tenth the 100 cm H2O rise in intravesical pressure during a hard cough. Thus exercise may be of limited help in alleviating stress incontinence when urethral resting pressure has been reduced too much, especially if the woman participates in activities that cause large increases in intraabdominal pressure.
INTEGRATION OF THE STRESS CONTINENCE CONTROL SYSTEM
The levator ani muscles, endopelvic fascia, and muscular structures of the urethra comprise a system. These muscles are recruited during a cough to help prevent urine loss during stress. The coordinated action of these elements depends upon the central nervous system.
Recent evidence has shown that nerve dysfunction accompanies stress incontinence. This is supported by the observation that many women, simply by learning to time a pelvic muscle contraction to occur during a cough, are able to eliminate stress incontinence during that cough (20, 21). The neural factors controlling this are poorly known but the implications for clinical practice are clear. Women need to be told when best to contract their pelvic muscles to prevent leakage, as well as learning to strengthen them. A stronger muscle that is not activated during the time of a cough cannot prevent stress incontinence. Therefore, teaching proper pelvic muscle timing is critical.
BIRTH-RELATED INJURY
Studies of the effect of vaginal birth on the sphincter mechanism reveal decreases in urethral closure pressure that are known to occur as a result of vaginal birth (31–33). These decreases have been ascribed to damage to the pelvic nerves based on studies indicating delayed conduction in the pudendal nerve (27, 28), as well as single fiber recordings showing that patients with SUI have had denervation of the pelvic musculature (29). The role of vaginal birth in this denervation process has been clarified by Allen’s EMG studies performed before and after vaginal birth (34). Allen found that most women after vaginal birth (but not cesarean section) have indications of neurologic injury as evidenced by increased motor unit potential (MUP) and increasing amounts of damage correlated with more evidence of SUI. Among a group with less than 120% change in MUP, when antepartum values were compared with postpartum measures in the same women, only 34% had SUI; while among the five women with MUP >120% all were incontinent (34). Recent MR studies are beginning to identify the anatomic structures most susceptible to birth-related damage (19).
CONCLUSION
The stress continence control system is a complex network of sub-systems that each contributes to the overall goal of maintaining continence. Understanding the pathophysiology of incontinence on the anatomical level will help to lead to identification of specific defects, thus allowing us to provide individualized treatment to the incontinent patient.
Acknowledgments
This work was supported by Public Health Service Grants R01 DK 47516 & 51405 and P30 AG 08808.
References
- 1.Herzog AR, Diokno AC, Brown MB, Normolle DP, Brock BM. Two-year incidence, remission, and change patterns of urinary incontinence in noninstitutionalized older adults. J Gerontol. 1990;45:M67–74. doi: 10.1093/geronj/45.2.m67. [DOI] [PubMed] [Google Scholar]
- 2.Thomas TM, Plymat KR, Blannin J, Meade TW. Prevalence of urinary incontinence. Br Med J. 1980;281:143–5. doi: 10.1136/bmj.281.6250.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diokno AC, Wells TJ, Brink CA. Urinary incontinence in elderly women: urodynamic evaluation. J Am Ger Soc. 1987;35:940–6. doi: 10.1111/j.1532-5415.1987.tb02296.x. [DOI] [PubMed] [Google Scholar]
- 4.Bergman A, Giovanni E. Three surgical procedures for genuine stress incontinence: five-year follow-up of a prospective randomized study. Am J Obstet Gynecol. 1995;173:66–71. doi: 10.1016/0002-9378(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 5.Colombo M, Scalambrino S, Maggioni A, Milani R. Gynecology: Burch colposuspension versus modified Marshal-Marchetti-Krantz urethropexy for primary genuine stress urinary incontinence: a prospective, randomized clinical trial. Am J Obstet Gynecol. 1994;171:573–9. doi: 10.1016/0002-9378(94)90404-9. [DOI] [PubMed] [Google Scholar]
- 6.Kim K-J, Ashton-Miller JA, Strohbehn K, DeLancey JOL, Schultz AB. The vesico-urethral pressuregram analysis of urethral function under stress. J Biomech. 1997;30:19–25. doi: 10.1016/s0021-9290(97)81291-2. [DOI] [PubMed] [Google Scholar]
- 7.DeLancey JOL. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol. 1994;170:1713–23. doi: 10.1016/s0002-9378(94)70346-9. [DOI] [PubMed] [Google Scholar]
- 8.Critchley HOD, Dixon JS, Gosling JA. Comparative study of the periurethral and perianal parts of the human levator ani muscle. Urol Int. 1980;35:226–32. doi: 10.1159/000280326. [DOI] [PubMed] [Google Scholar]
- 9.DeLancey JO, Hurd WW. Size of the urogenital hiatus in the levator ani muscles in normal women and women with pelvic organ prolapse. Obstet Gynecol. 1998;91:364–8. doi: 10.1016/s0029-7844(97)00682-0. [DOI] [PubMed] [Google Scholar]
- 10.Howard D, Miller JM, DeLancey JOL, Ashton-Miller JA. Differential effects of cough, valsalva and continence status on vesical neck movement. Am J Obstet Gynecol. 2000;95:535–40. doi: 10.1016/s0029-7844(99)00618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson AC, Edmonds PB, Williams NL. Treatment of stress urinary incontinence due to paravaginal fascial defect. Obstet Gynecol. 1981;57:357–62. [PubMed] [Google Scholar]
- 12.DeLancey JOL. Anatomy and physiology of urinary incontinence. Clin Obstet Gynecol. 1990;33:298–307. doi: 10.1097/00003081-199006000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Sampselle CM, Miller JM, Mims B, DeLancey JOL, Ashton-Miller JA, Antonakis CL. Pelvic muscle exercise reduces transient incontinence during pregnancy and postpartum. Obstet Gynecol. 1998;91:406–12. doi: 10.1016/s0029-7844(97)00672-8. [DOI] [PubMed] [Google Scholar]
- 14.Howard D, DeLancey JOL, Tunn R, Ashton-Miller JA. Racial differences in the structure and function of the stress urinary continence mechanism. Obstet Gynecol. 2000;95:713–7. doi: 10.1016/s0029-7844(00)00786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thelen DG, Schultz AB, Alexander NB, Ashton-Miller JA. Effects of age on rapid ankle torque development. J Gerontol: Med Sci. 1996;51A:M226–32. doi: 10.1093/gerona/51a.5.m226. [DOI] [PubMed] [Google Scholar]
- 16.Thelen DG, Ashton-Miller JA, Schultz AB, Alexander NB. Do neural factors underlie age differences in rapid ankle torque development? J Am Ger Soc. 1996;44:804–8. doi: 10.1111/j.1532-5415.1996.tb03737.x. [DOI] [PubMed] [Google Scholar]
- 17.Sinkjaer T, Toft E, Andreassen S, Hornemann B. Muscle stiffness in human ankle dorsiflexors: intrinsic and reflex components. J Neurophysiol. 1988;60:1110–21. doi: 10.1152/jn.1988.60.3.1110. [DOI] [PubMed] [Google Scholar]
- 18.Klutke GC, Golomb J, Barbaric Z, Raz S. The anatomy of stress incontinence: Magnetic resonance imaging of the female bladder neck and urethra. J Urol. 1990;143:563–6. doi: 10.1016/s0022-5347(17)40020-6. [DOI] [PubMed] [Google Scholar]
- 19.Tunn R, DeLancey JOL, Howard D, Thorp JM, Ashton-Miller JA, Quint LE. MR imaging of levator ani muscle recovery following vaginal delivery. Int Urogynecol J. 1999;10:300–7. doi: 10.1007/s001929970006. [DOI] [PubMed] [Google Scholar]
- 20.Miller JM, Ashton-Miller JA, DeLancey JOL. Quantification of cough-related urine loss using the paper towel test. Obstet Gynecol. 1998;91:705–9. doi: 10.1016/s0029-7844(98)00045-3. [DOI] [PubMed] [Google Scholar]
- 21.Miller JM, Ashton-Miller JA, DeLancey JOL. A pelvic muscle precontraction can reduce cough-related urine loss in selected women with mild SUI. J Am Geriatr Soc. 1998;46:870–4. doi: 10.1111/j.1532-5415.1998.tb02721.x. [DOI] [PubMed] [Google Scholar]
- 22.Strohbehn K, Quint LE, Wojno EJ, DeLancey JOL. MRI anatomy of the female urethra: a direct histologic comparison. Obstet Gynecol. 1996;88:750–6. doi: 10.1016/0029-7844(96)00323-7. [DOI] [PubMed] [Google Scholar]
- 23.Rud T, Anderson KE, Asmussen M, Hunting A, Ulmsten U. Factors maintaining the intraurethral pressure in women. Invest Urol. 1980;17:343–7. [PubMed] [Google Scholar]
- 24.DeLancey JOL. Correlative study of paraurethral anatomy. Obstet Gynecol. 1986;68:91–7. [PubMed] [Google Scholar]
- 25.Gosling JA, Dixon JS, Critchley HOD, Thompson SA. A comparative study of the human external sphincter and periurethral levator ani muscles. Br J Urol. 1981;53:35–41. doi: 10.1111/j.1464-410x.1981.tb03125.x. [DOI] [PubMed] [Google Scholar]
- 26.Hilton P, Stanton SL. Urethral pressure measurement by microtransducer: the results in symptom-free and in those with genuine stress incontinence. Br J Obstet Gynaecol. 1983;90:919–33. doi: 10.1111/j.1471-0528.1983.tb06764.x. [DOI] [PubMed] [Google Scholar]
- 27.Snooks SJ, Swash M, Henry MM, Setchell M. Risk factors in childbirth causing damage to the pelvic floor innervation. Int J Colorectal Dis. 1986;1:20–4. doi: 10.1007/BF01648831. [DOI] [PubMed] [Google Scholar]
- 28.Smith ARB, Hosker GL, Warrell DW. The role of partial denervation of the pelvic floor in the aetiology of genitourinary prolapse and stress incontinence of urine: a neurophysiological study. Br J Obstet Gynecol. 1989;96:24–8. doi: 10.1111/j.1471-0528.1989.tb01571.x. [DOI] [PubMed] [Google Scholar]
- 29.Smith ARB, Hosker GL, Warrell DW. The role of pudendal nerve damage in the aetiology of genuine stress incontinence in women. Br J Obstet Gynaecol. 1989;96:29–32. doi: 10.1111/j.1471-0528.1989.tb01572.x. [DOI] [PubMed] [Google Scholar]
- 30.Skeleton DA, Young A, Greig CA, Malbut KE. Effects of resistance training on strength, power, and selected functional abilities of women aged 75 and older. J Am Geriatric Soc. 1995;43:1081–7. doi: 10.1111/j.1532-5415.1995.tb07004.x. [DOI] [PubMed] [Google Scholar]
- 31.Iosif S, Ulmsten U. Comparative urodynamic studies of continent and stress incontinent women in pregnancy and in the puerperium. Am J Obstet Gynecol. 1981;140:645–50. doi: 10.1016/0002-9378(81)90197-6. [DOI] [PubMed] [Google Scholar]
- 32.Iosif S. Stress incontinence during pregnancy and in puerperium. Int J Gynaecol Obstet. 1981;19:13–20. doi: 10.1016/0020-7292(81)90033-3. [DOI] [PubMed] [Google Scholar]
- 33.van Geelen JM, Lemmens WA, Eskes TK, Martin CB., Jr The urethral pressure profile in pregnancy and after delivery in healthy nulliparous women. Am J Obstet-Gynecol. 1982;144:636–49. doi: 10.1016/0002-9378(82)90431-8. [DOI] [PubMed] [Google Scholar]
- 34.Allen RE, Hosker GL, Smith ARB, Warrell DW. Pelvic floor damage and childbirth: a neurophysiological study. Br J Obstet Gynaecol. 1990;97:770–9. doi: 10.1111/j.1471-0528.1990.tb02570.x. [DOI] [PubMed] [Google Scholar]


