Abstract
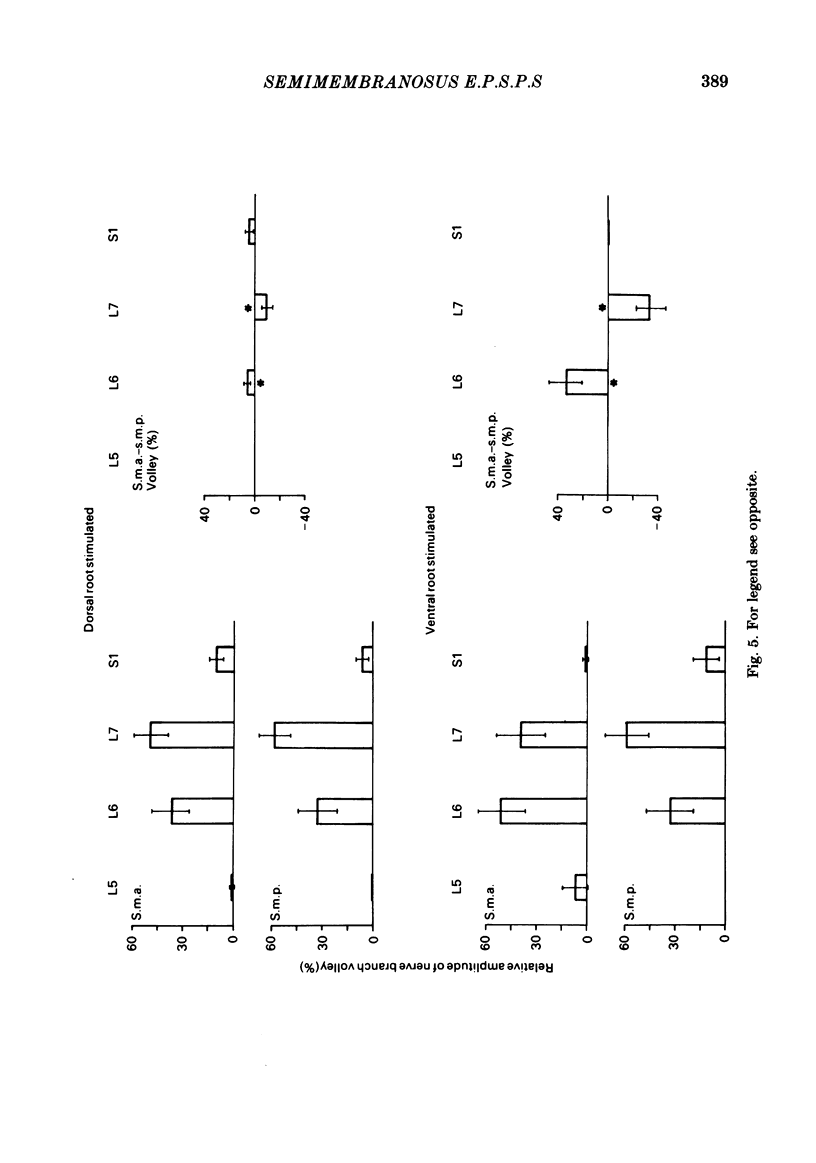
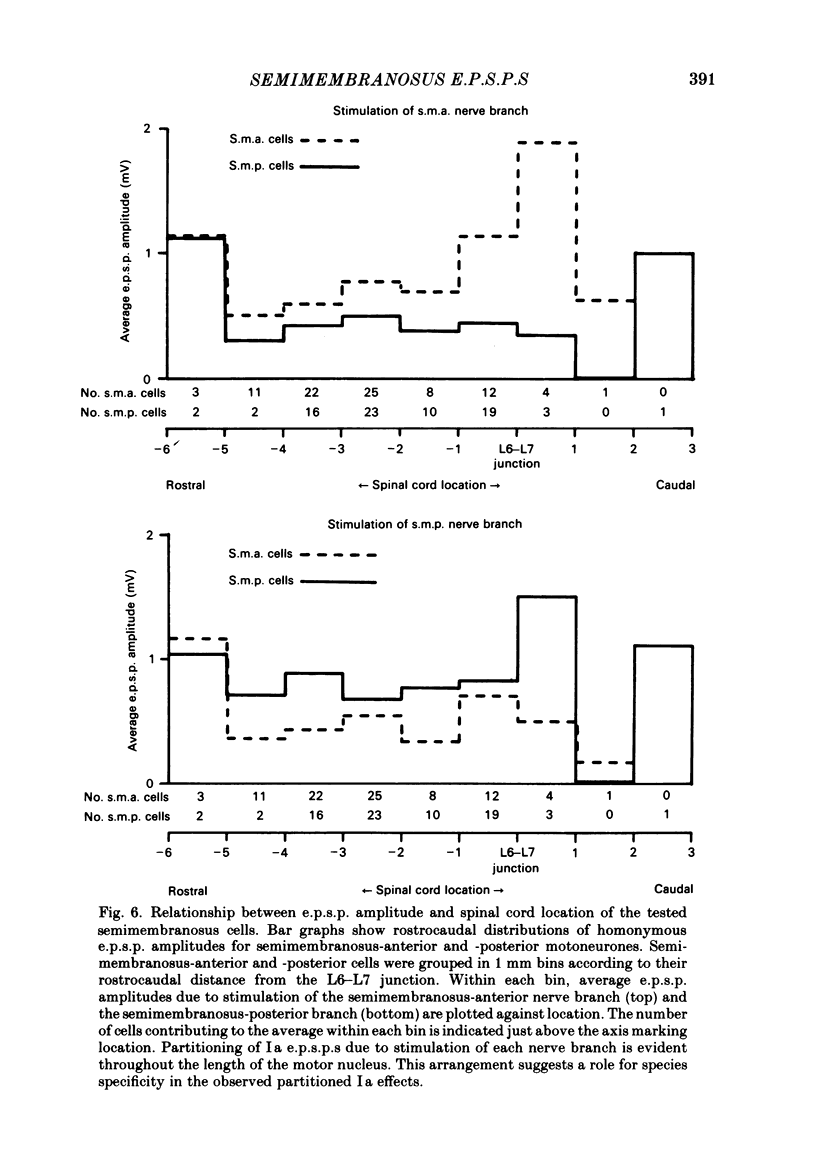
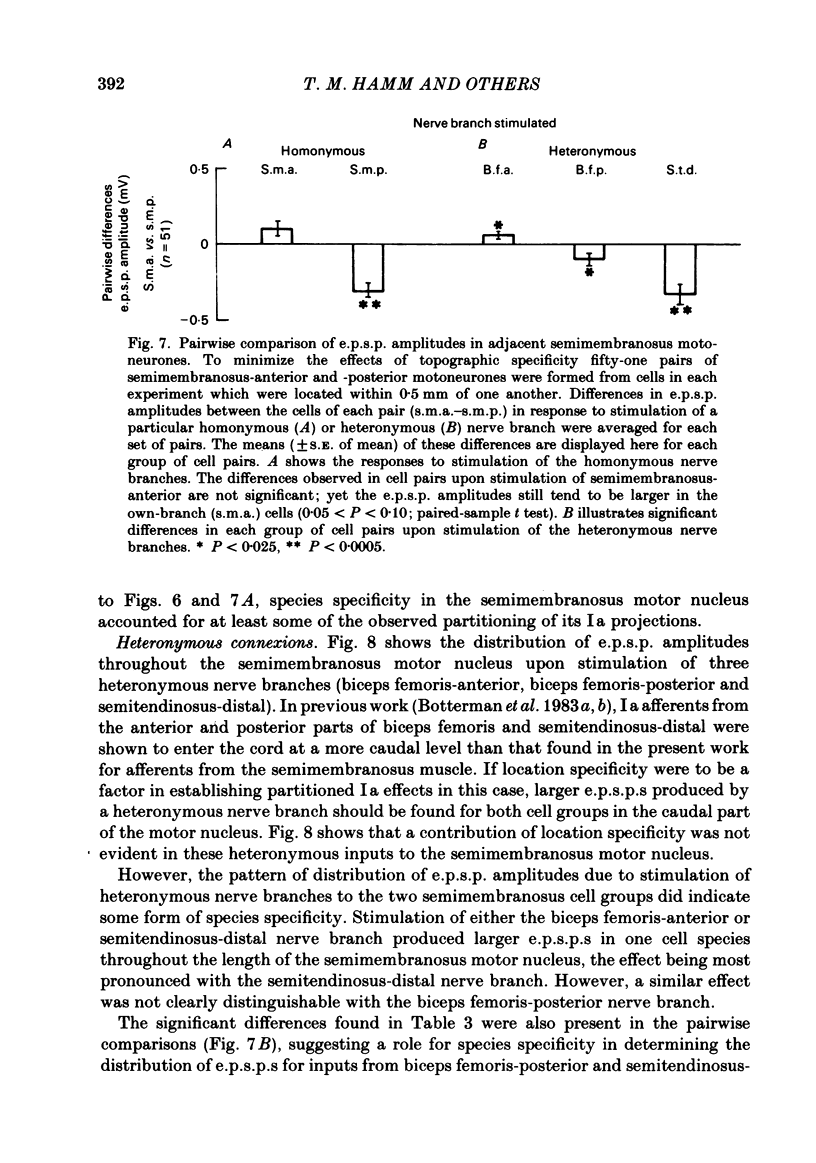
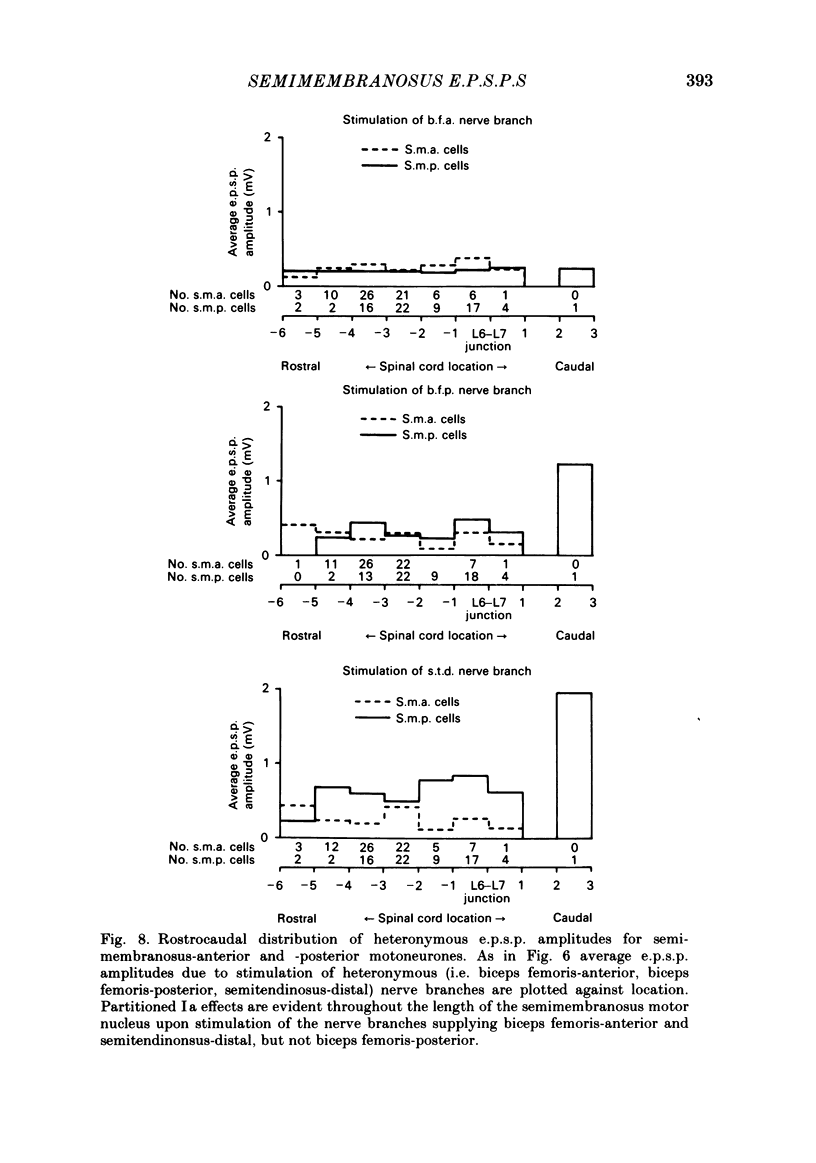
In anaesthetized low-spinal cats, intracellular recordings were made of the Ia excitatory post-synaptic potential (e.p.s.p.) responses of semimembranosus motoneurones to electrical stimulation (Group I range) of nerve branches supplying the anterior and posterior heads of semimembranosus, the anterior and posterior parts of biceps femoris, and the distal part of semitendinosus. Recordings were also made during stimulation of nerves to the gracilis muscle and to the vasti muscle group. Stimulation of the semimembranosus-anterior nerve branch produced Ia e.p.s.p.s. of greater amplitude in semimembranosus-anterior motoneurones than in semimembranosus-posterior cells; likewise, stimulation of the semimembranosus-posterior nerve branch produced larger e.p.s.p.s. in cells which supplied the posterior head than in those which supplied the anterior head. Stimulation of the nerve branches to components of two 'flexor' muscles (Sherrington, 1910), biceps-posterior and semitendinosus-distal, produced larger e.p.s.p.s in semimembranosus-posterior cells than in the anterior motoneurones. A tendency was found for stimulation of the nerve to biceps femoris-anterior (an 'extensor') to produce larger e.p.s.p.s in semimembranosus-anterior than in-posterior motoneurones. However, this effect was of borderline (0.06 greater than P greater than 0.05) significance. The limited monosynaptic input produced by stimulation of the nerves to the gracilis and vasti muscles showed that their Ia axons do not distinguish between the two semimembranosus cell groups. A slight topographic organization of motoneurones within the semimembranosus motor nucleus was found, with anterior cells encountered, on average, at a more rostral level of the spinal cord than posterior cells. A similar topographic arrangement was observed in the rostrocaudal distribution of Group I afferent fibres in the dorsal roots and motor axons from the two sets of motoneurones in the ventral roots. These findings are consistent with 'location specificity' (Scott & Mendell, 1976) being a factor which contributes to the observed pattern of homonymous Ia connexions. A role for 'species specificity' (Scott & Mendell, 1976) in determining the observed pattern of homonymous Ia connexions was indicated by species-dependent differences in e.p.s.p. amplitude in pairs of semimembranosus-anterior and -posterior motoneurones at similar rostrocaudal locations in the spinal cord. The pattern of heteronymous connexions to the semimembranosus motor nucleus also showed evidence for species specificity. However, no clear topographic pattern was evident in these connexions.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Barone F. C., Wayner M. J., Aguilar-Baturoni H. U., Guevara-Aguilar R. A bipolar electrode for peripheral nerve stimulation. Brain Res Bull. 1979 May-Jun;4(3):421–422. doi: 10.1016/s0361-9230(79)80019-2. [DOI] [PubMed] [Google Scholar]
- Barrett J. N., Crill W. E. Specific membrane properties of cat motoneurones. J Physiol. 1974 Jun;239(2):301–324. doi: 10.1113/jphysiol.1974.sp010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilotto G., Schor R. H., Uchino Y., Wilson V. J. Localization of proprioceptive reflexes in the splenius muscle of the cat. Brain Res. 1982 Apr 22;238(1):217–221. doi: 10.1016/0006-8993(82)90786-7. [DOI] [PubMed] [Google Scholar]
- Bodine S. C., Roy R. R., Meadows D. A., Zernicke R. F., Sacks R. D., Fournier M., Edgerton V. R. Architectural, histochemical, and contractile characteristics of a unique biarticular muscle: the cat semitendinosus. J Neurophysiol. 1982 Jul;48(1):192–201. doi: 10.1152/jn.1982.48.1.192. [DOI] [PubMed] [Google Scholar]
- Botterman B. R., Hamm T. M., Reinking R. M., Stuart D. G. Distribution of monosynaptic Ia excitatory post-synaptic potentials in the motor nucleus of the cat semitendinosus muscle. J Physiol. 1983 May;338:379–393. doi: 10.1113/jphysiol.1983.sp014678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botterman B. R., Hamm T. M., Reinking R. M., Stuart D. G. Localization of monosynaptic Ia excitatory post-synaptic potentials in the motor nucleus of the cat biceps femoris muscle. J Physiol. 1983 May;338:355–377. doi: 10.1113/jphysiol.1983.sp014677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink E. E., Jinnai K., Wilson V. J. Pattern of segmental monosynaptic input to cat dorsal neck motoneurons. J Neurophysiol. 1981 Sep;46(3):496–505. doi: 10.1152/jn.1981.46.3.496. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN L. A. Localization of stretch reflex. J Neurophysiol. 1953 May;16(3):272–285. doi: 10.1152/jn.1953.16.3.272. [DOI] [PubMed] [Google Scholar]
- COHEN L. A. Organization of stretch reflex into two types of direct spinal arcs. J Neurophysiol. 1954 Sep;17(5):443–453. doi: 10.1152/jn.1954.17.5.443. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol. 1958 Dec 4;144(2):271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Lundberg A. An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiol Scand. 1969 Apr;75(4):614–630. doi: 10.1111/j.1748-1716.1969.tb04415.x. [DOI] [PubMed] [Google Scholar]
- Fleshman J. W., Munson J. B., Sypert G. W., Friedman W. A. Rheobase, input resistance, and motor-unit type in medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1981 Dec;46(6):1326–1338. doi: 10.1152/jn.1981.46.6.1326. [DOI] [PubMed] [Google Scholar]
- Hamm T. M., Botterman B. R., Reinking R. M., Stuart D. G. Characteristics of M spikes in cat motoneurons and their significance for the measurement of small composite Ia EPSPs. Exp Brain Res. 1983;49(1):68–76. doi: 10.1007/BF00235542. [DOI] [PubMed] [Google Scholar]
- Lucas S. M., Cope T. C., Binder M. D. Analysis of individual Ia-afferent EPSPs in a homonymous motoneuron pool with respect to muscle topography. J Neurophysiol. 1984 Jan;51(1):64–74. doi: 10.1152/jn.1984.51.1.64. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Henneman E. Topographic distribution of terminals of Ia and group II fibers in spinal cord, as revealed by postsynaptic population potentials. J Neurophysiol. 1980 Apr;43(4):968–985. doi: 10.1152/jn.1980.43.4.968. [DOI] [PubMed] [Google Scholar]
- McDonagh J. C., Binder M. D., Reinking R. M., Stuart D. G. A commentary on muscle unit properties in cat hindlimb muscles. J Morphol. 1980 Nov;166(2):217–230. doi: 10.1002/jmor.1051660208. [DOI] [PubMed] [Google Scholar]
- McKeon B., Gandevia S., Burke D. Absence of somatotopic projection of muscle afferents onto motoneurons of same muscle. J Neurophysiol. 1984 Feb;51(2):185–194. doi: 10.1152/jn.1984.51.2.185. [DOI] [PubMed] [Google Scholar]
- Munson J. B., Fleshman J. W., Zengel J. E., Sypert G. W. Synaptic and mechanical coupling between type-identified motor units and individual spindle afferents of medial gastrocnemius muscle of the cat. J Neurophysiol. 1984 Jun;51(6):1268–1283. doi: 10.1152/jn.1984.51.6.1268. [DOI] [PubMed] [Google Scholar]
- Nelson S. G., Mendell L. M. Projection of single knee flexor Ia fibers to homonymous and heteronymous motoneurons. J Neurophysiol. 1978 May;41(3):778–787. doi: 10.1152/jn.1978.41.3.778. [DOI] [PubMed] [Google Scholar]
- Peters S. E., Rick C. The actions of three hamstring muscles of the cat: a mechanical analysis. J Morphol. 1977 Jun;152(3):315–328. doi: 10.1002/jmor.1051520304. [DOI] [PubMed] [Google Scholar]
- ROMANES G. J. The motor cell columns of the lumbo-sacral spinal cord of the cat. J Comp Neurol. 1951 Apr;94(2):313–363. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- Scott J. G., Mendell L. M. Individual EPSPs produced by single triceps surae Ia afferent fibers in homonymous and heteronymous motoneurons. J Neurophysiol. 1976 Jul;39(4):679–692. doi: 10.1152/jn.1976.39.4.679. [DOI] [PubMed] [Google Scholar]
- Sherrington C. S. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol. 1910 Apr 26;40(1-2):28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer E. K., Watt D. G. Referencing procedure for location of lumbosacral alpha-motoneurons. Pflugers Arch. 1976 Nov 5;366(2-3):269–271. doi: 10.1007/BF00585889. [DOI] [PubMed] [Google Scholar]
- Stein R. B., Nichols T. R., Jhamandas J., Davis L., Charles D. Stable long-term recordings from cat peripheral nerves. Brain Res. 1977 Jun 3;128(1):21–38. doi: 10.1016/0006-8993(77)90233-5. [DOI] [PubMed] [Google Scholar]
- Watt D. G., Stauffer E. K., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 1. Spindle primary and tendon organ afferents. J Neurophysiol. 1976 Nov;39(6):1375–1392. doi: 10.1152/jn.1976.39.6.1375. [DOI] [PubMed] [Google Scholar]
- Zajac F. E. Thigh muscle activity during maximum-height jumps by cats. J Neurophysiol. 1985 Apr;53(4):979–994. doi: 10.1152/jn.1985.53.4.979. [DOI] [PubMed] [Google Scholar]
- Zengel J. E., Reid S. A., Sypert G. W., Munson J. B. Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1985 May;53(5):1323–1344. doi: 10.1152/jn.1985.53.5.1323. [DOI] [PubMed] [Google Scholar]


