Abstract
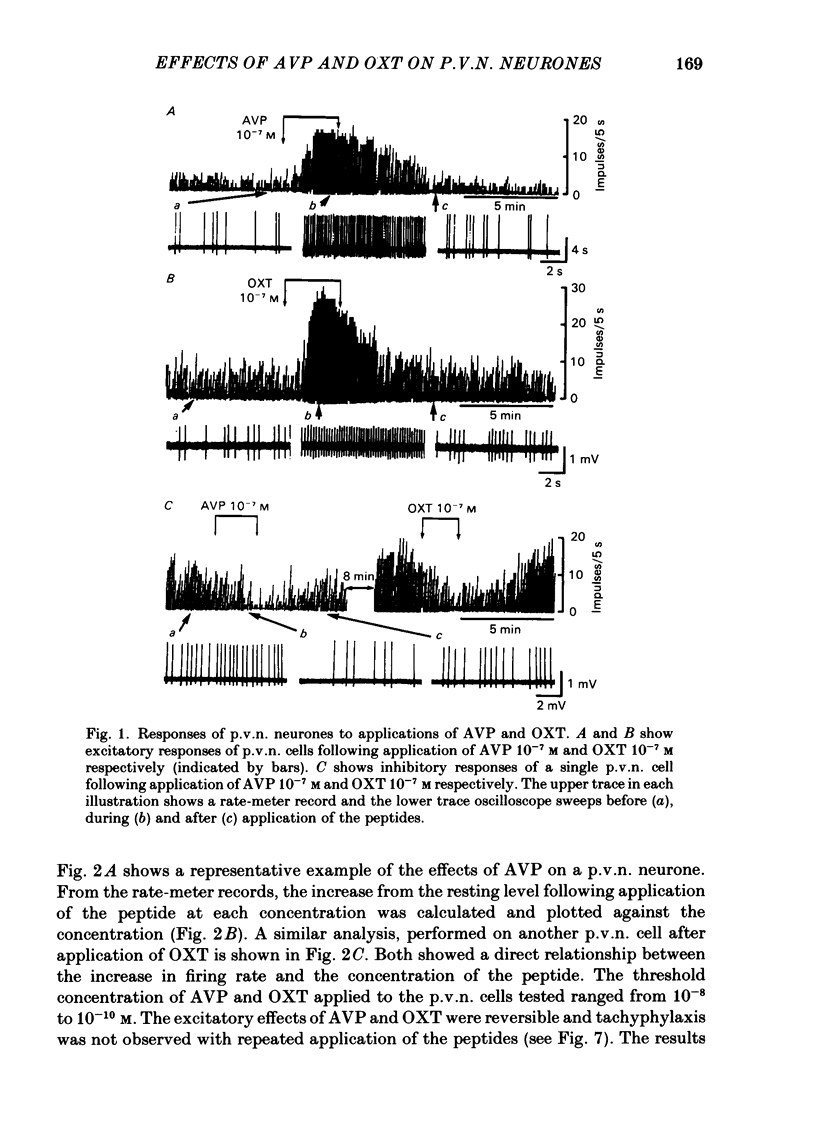
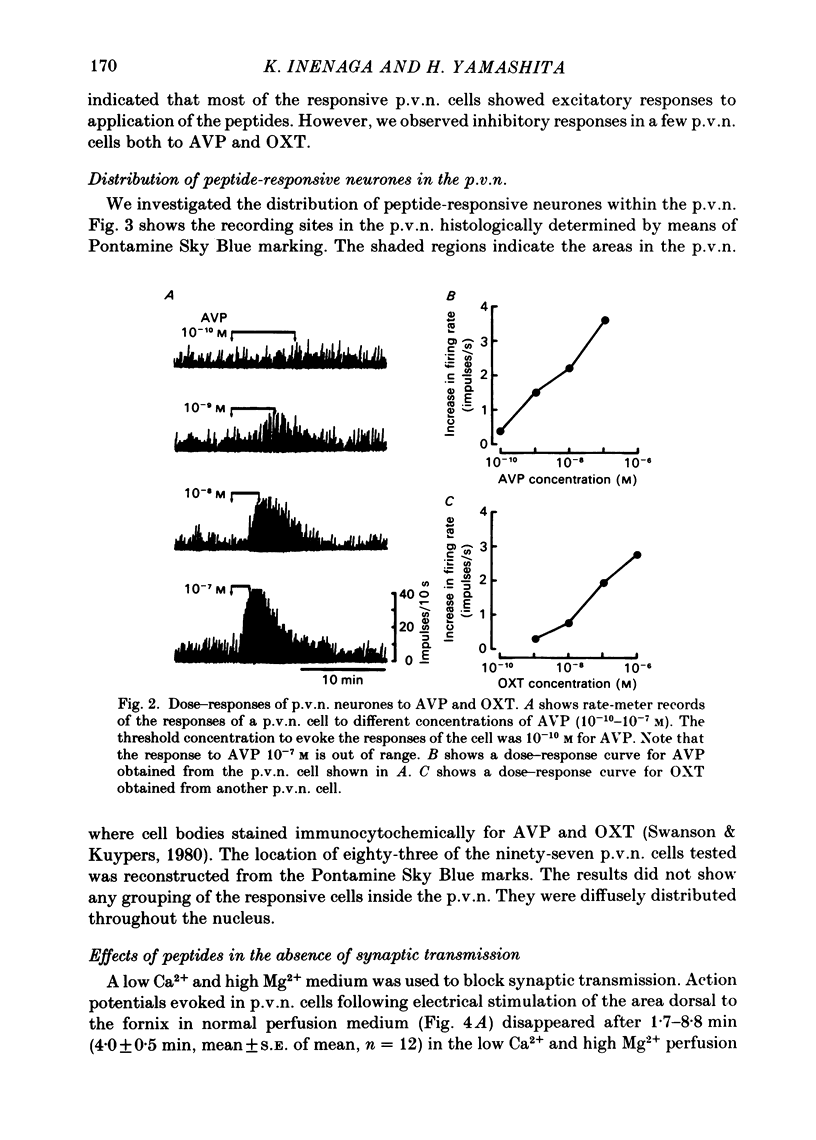
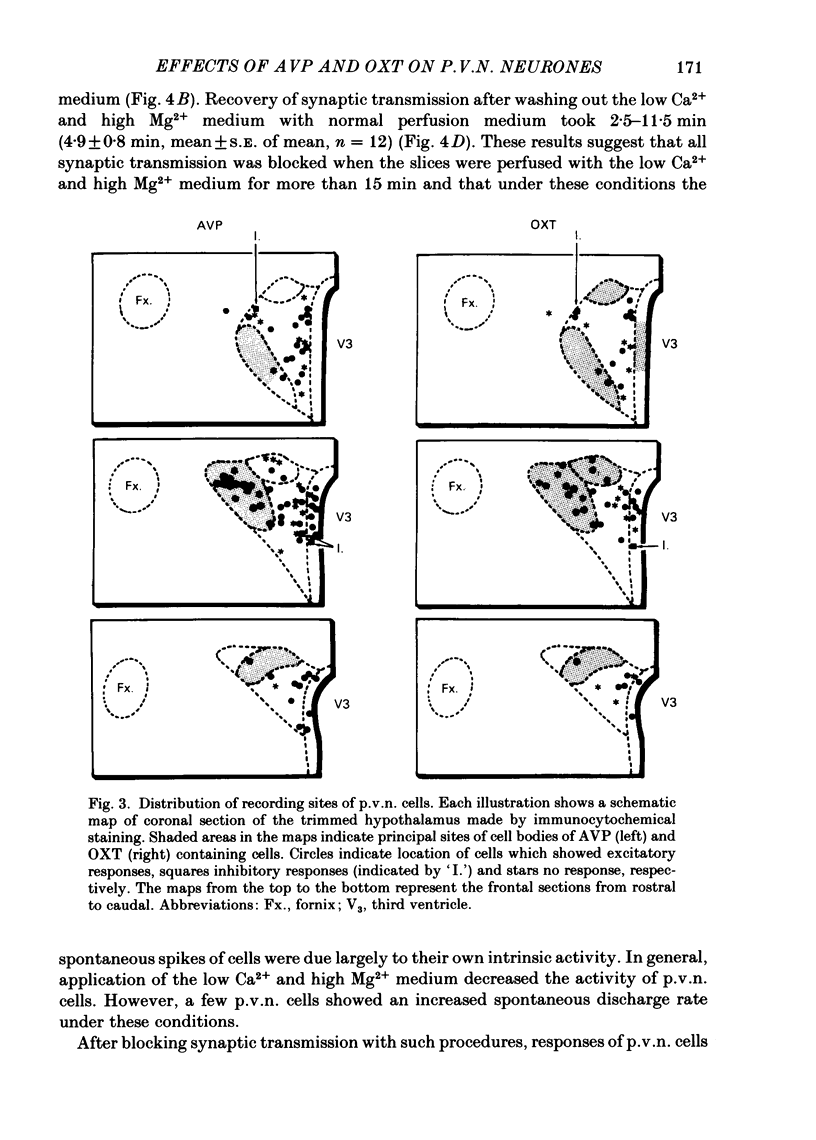
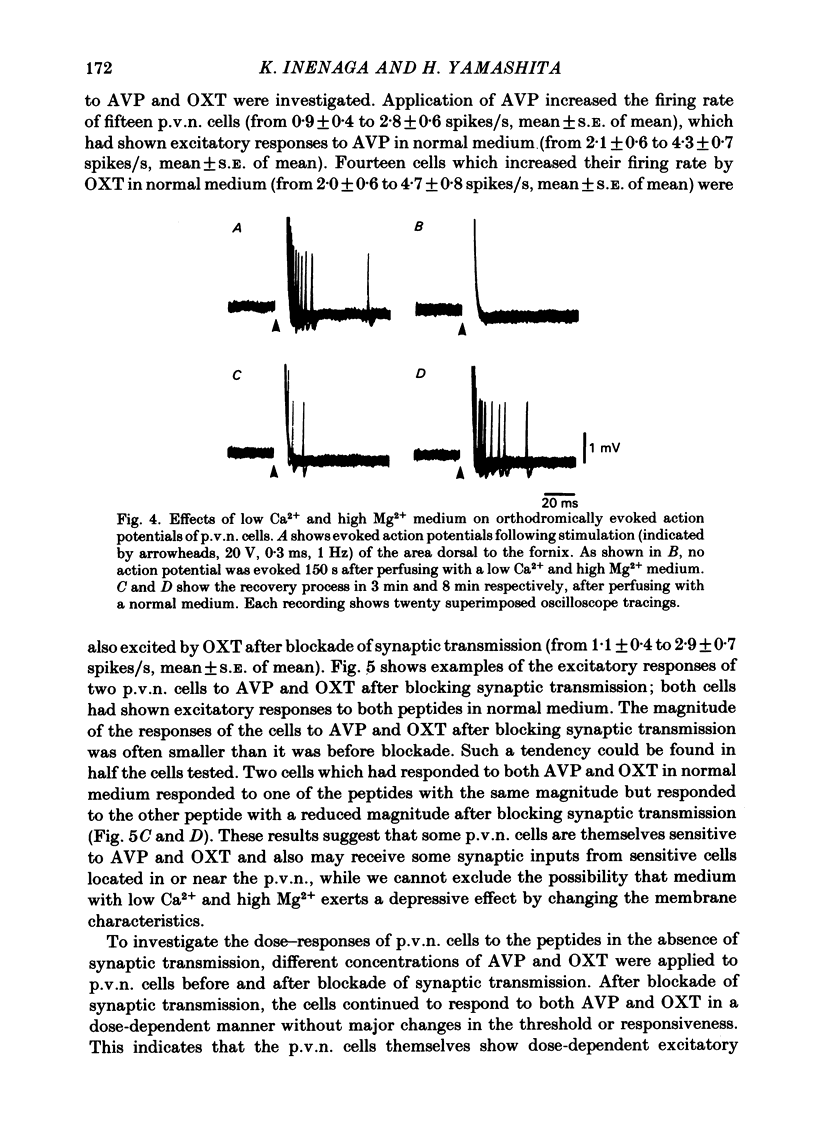
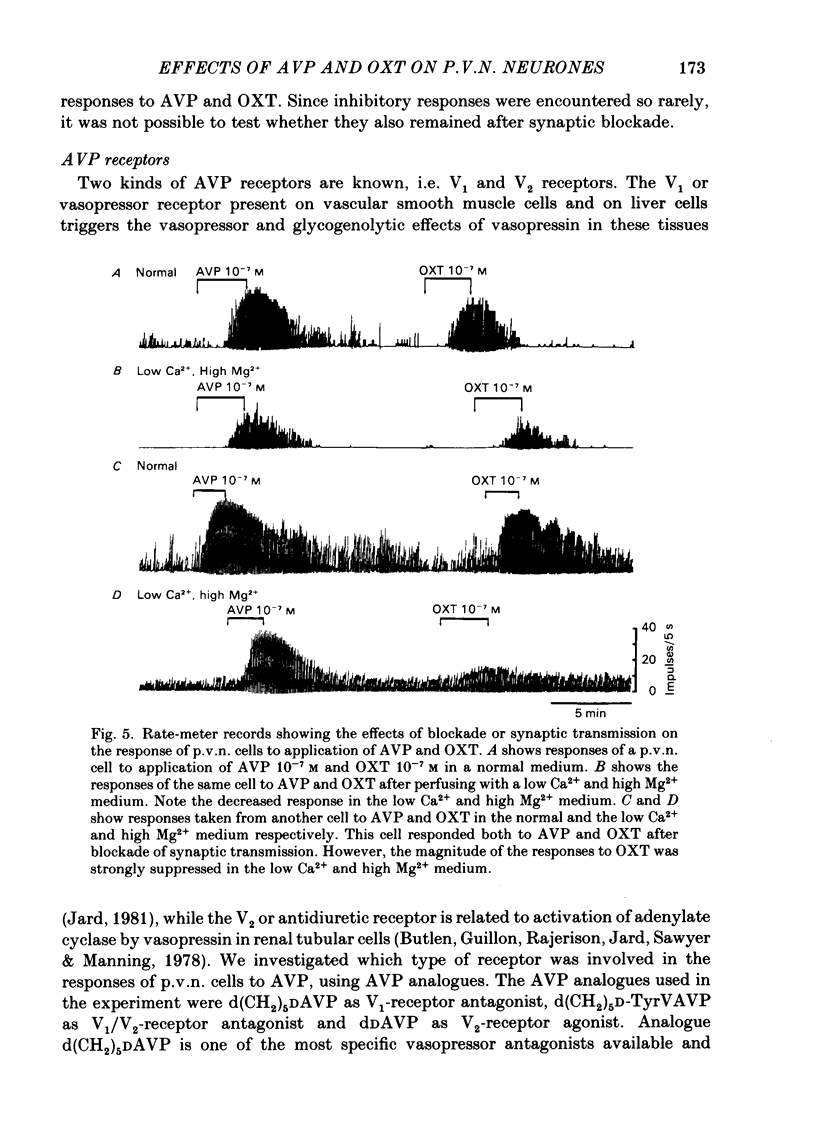
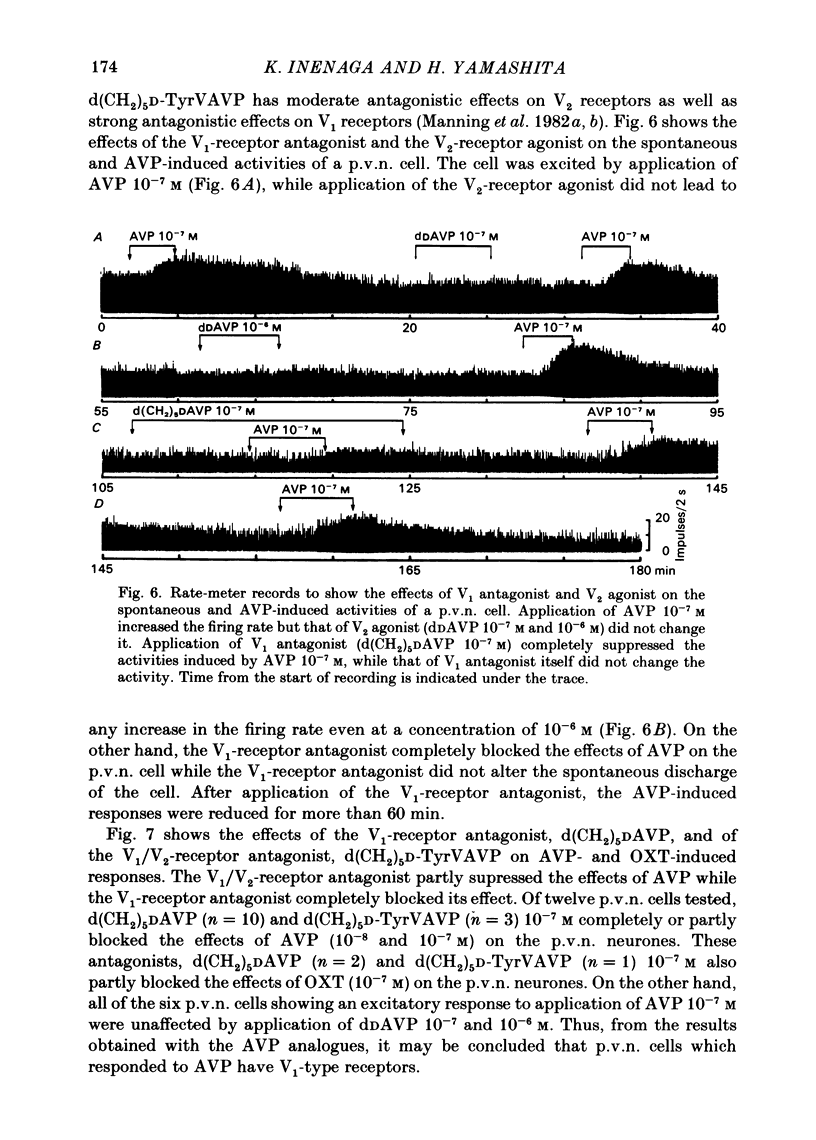
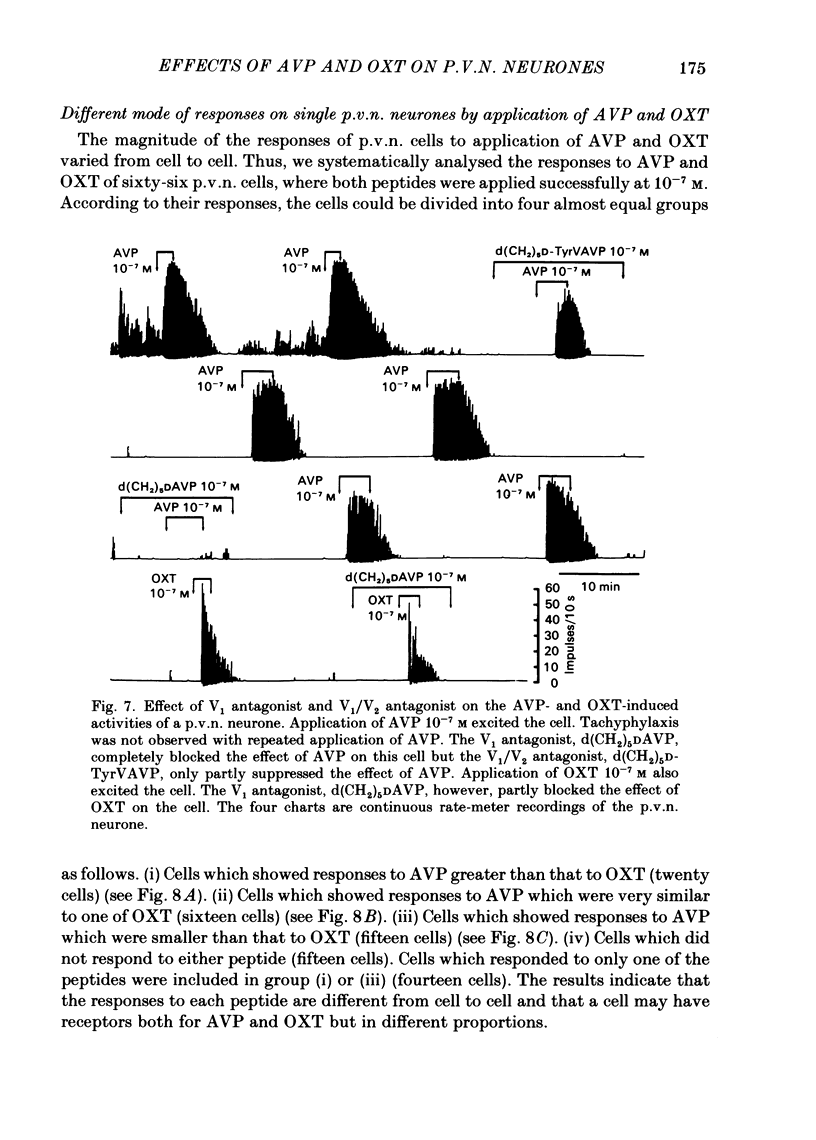
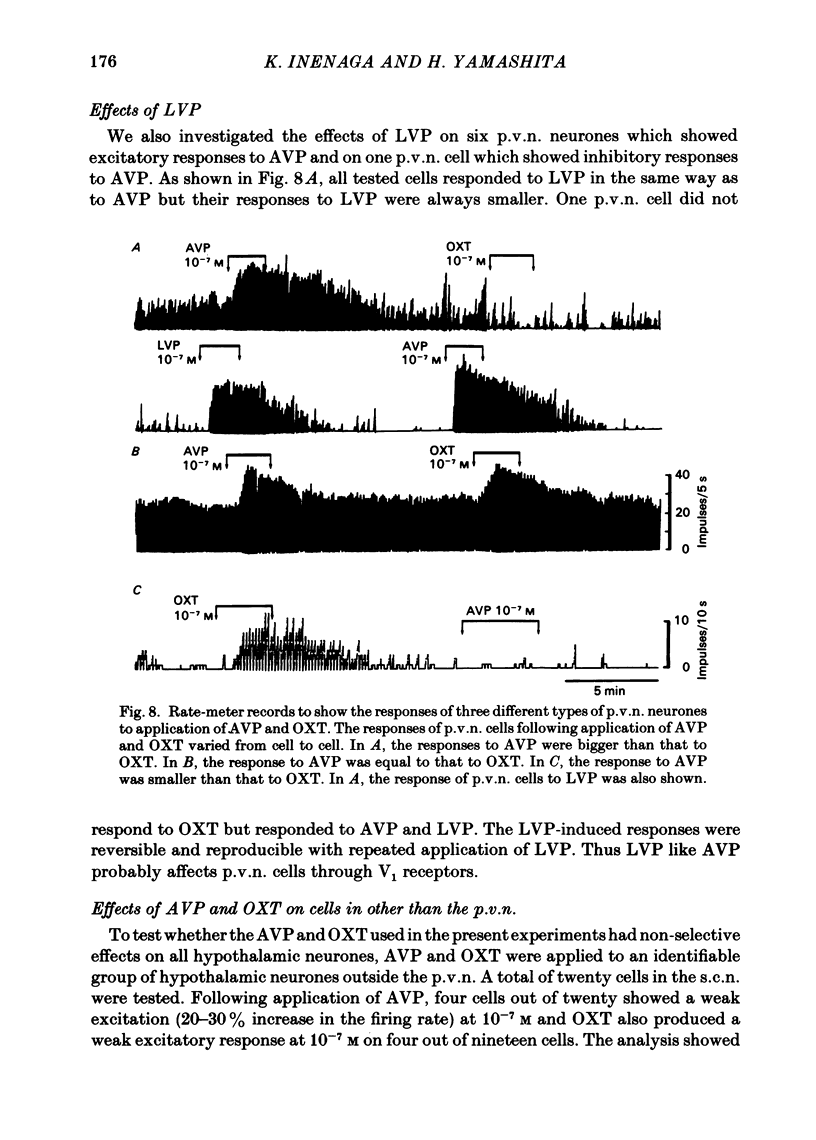
Extracellular recordings were made from ninety-seven spontaneously firing cells in the paraventricular nucleus (p.v.n.) of the rat hypothalamic slice preparation. The spontaneously firing cells tested fired at 0.1-8 spikes/s but the majority showed a slow irregular firing pattern. The average firing rate of all ninety-seven cells was 2.2 +/- 0.2 spikes/s (mean +/- S.E. of mean). Six cells showed a phasic firing pattern. Following bath application of arginine-vasopressin (AVP) 10(-7) M, sixty-four (66%) of ninety-seven p.v.n. cells showed excitatory responses and three (3%) cells inhibitory responses. Bath application of oxytocin (OXT) 10(-7) M excited thirty-nine (57%) of sixty-eight p.v.n. cells and inhibited two (3%) cells. Individual p.v.n. cells responded to application of both AVP and OXT, but the magnitude and threshold of the responses varied from cell to cell. Of the sixty-six cells tested with both peptides at 10(-7) M, sixteen showed similar responses to both and fifteen showed no response to either: twenty cells showed a greater response to AVP and fifteen a greater response to OXT. Of six phasic firing cells, two showed excitatory responses to AVP and all four cells tested did not show any response to OXT. The dose-dependence of the response to AVP and OXT was tested in six p.v.n. cells. There was a direct relationship between peptide concentration and increased firing rate. The threshold concentration of the peptides ranged from 10(-8) to 10(-10) M. The cells responsive to the peptides were not located in particular areas of the p.v.n. but were diffusely distributed throughout the nucleus. After blocking synaptic transmission with a low Ca2+ and high Mg2+ medium, all tested cells (AVP, n = 15; OXT, n = 14) which had responded to applications of AVP or OXT in normal medium still showed responses to the peptides, although the effect was less marked in half the cells. However, in the absence of synaptic transmission two cells showed unimpaired responses to one of the peptides but greatly depressed responses to the other. The V1-receptor antagonist [1-(beta-mercapto-, beta-cyclopentamethylenepropionic acid)], 8-D-arginine-vasopressin (d(CH2)5DAVP) or V1/V2-receptor antagonist [1-(beta-mercapto-, beta-cyclopentamethylenepropionic acid), 2-D-tyrosine,4-valine]arginine-vasopressin (d(CH2)5D-TyrVAVP) completely or partly blocked the AVP-induced responses, while the V2-receptor agonist 1-deamino-8-D-arginine-vasopressin (dDAVP) did not influence the spontaneous discharges of the cells.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe H., Inoue M., Matsuo T., Ogata N. The effects of vasopressin on electrical activity in the guinea-pig supraoptic nucleus in vitro. J Physiol. 1983 Apr;337:665–685. doi: 10.1113/jphysiol.1983.sp014648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaishi T., Ellendorff F. Electrical properties of paraventricular neurosecretory neurons with and without recurrent inhibition. Brain Res. 1983 Feb 28;262(1):151–154. doi: 10.1016/0006-8993(83)90479-1. [DOI] [PubMed] [Google Scholar]
- Biegon A., Terlou M., Voorhuis T. D., de Kloet E. R. Arginine-vasopressin binding sites in rat brain: a quantitative autoradiographic study. Neurosci Lett. 1984 Feb 24;44(3):229–234. doi: 10.1016/0304-3940(84)90027-2. [DOI] [PubMed] [Google Scholar]
- Buijs R. M. Immunocytochemical demonstration of vasopressin and oxytocin in the rat brain by light and electron microscopy. J Histochem Cytochem. 1980 Apr;28(4):357–360. doi: 10.1177/28.4.6989899. [DOI] [PubMed] [Google Scholar]
- Buijs R. M., Van Heerikhuize J. J. Vasopressin and oxytocin release in the brain--a synaptic event. Brain Res. 1982 Dec 2;252(1):71–76. doi: 10.1016/0006-8993(82)90979-9. [DOI] [PubMed] [Google Scholar]
- Butlen D., Guillon G., Rajerison R. M., Jard S., Sawyer W. H., Manning M. Structural requirements for activation of vasopressin-sensitive adenylate cyclase, hormone binding, and antidiuretic actions: effects of highly potent analogues and competitive inhibitors. Mol Pharmacol. 1978 Nov;14(6):1006–1017. [PubMed] [Google Scholar]
- Charpak S., Armstrong W. E., Mühlethaler M., Dreifuss J. J. Stimulatory action of oxytocin on neurones of the dorsal motor nucleus of the vagus nerve. Brain Res. 1984 May 21;300(1):83–89. doi: 10.1016/0006-8993(84)91342-8. [DOI] [PubMed] [Google Scholar]
- Dogterom J., Van Wimersma Greidanus T. B., Swabb D. F. Evidence for the release of vasopressin and oxytocin into cerebrospinal fluid: measurements in plasma and CSF of intact and hypophysectomized rats. Neuroendocrinology. 1977;24(2):108–118. doi: 10.1159/000122702. [DOI] [PubMed] [Google Scholar]
- Dudek F. E., Hatton G. I., Macvicar B. A. Intracellular recordings from the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1980 Apr;301:101–114. doi: 10.1113/jphysiol.1980.sp013192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball R. E., Dyer R. G. Plasma oxytocin concentration and paraventricular neurone activity in rats with diencephalic islands and intact brains. J Physiol. 1971 Jul;216(1):227–235. doi: 10.1113/jphysiol.1971.sp009520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbey M. P., Coote J. H., Fleetwood-Walker S., Peterson D. F. The influence of the paraventriculo-spinal pathway, and oxytocin and vasopressin on sympathetic preganglionic neurones. Brain Res. 1982 Nov 18;251(2):283–290. doi: 10.1016/0006-8993(82)90745-4. [DOI] [PubMed] [Google Scholar]
- Haller E. W., Wakerley J. B. Electrophysiological studies of paraventricular and supraoptic neurones recorded in vitro from slices of rat hypothalamus. J Physiol. 1980 May;302:347–362. doi: 10.1113/jphysiol.1980.sp013247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jard S. Les isorécepteurs de la vasopressine dans le foie et dans le rein: relation entre fixation d'hormone et réponse biologique. J Physiol (Paris) 1981;77(4-5):621–628. [PubMed] [Google Scholar]
- Kawata M., Ueda S., Sano Y. Two types of oxytocin and vasopressin nerve fibers in the intra- and extrahypothalamic neuronal systems as revealed by immunohistochemistry. Acta Anat (Basel) 1983;116(3):193–200. doi: 10.1159/000145742. [DOI] [PubMed] [Google Scholar]
- Koizumi K., Ishikawa T., Brooks C. M. The existence of facilitatory axon collaterals in neurosecretory cells of the hypothalamus. Brain Res. 1973 Dec 7;63:408–413. doi: 10.1016/0006-8993(73)90114-5. [DOI] [PubMed] [Google Scholar]
- Leng G., Mason W. T. Influence of vasopressin upon firing patterns of supraoptic neurons: a comparison of normal and Brattleboro rats. Ann N Y Acad Sci. 1982;394:153–158. doi: 10.1111/j.1749-6632.1982.tb37422.x. [DOI] [PubMed] [Google Scholar]
- Manning M., Lammek B., Kruszynski M., Seto J., Sawyer W. H. Design of potent and selective antagonists of the vasopressor responses to arginine-vasopressin. J Med Chem. 1982 Apr;25(4):408–414. doi: 10.1021/jm00346a015. [DOI] [PubMed] [Google Scholar]
- Manning M., Olma A., Klis W. A., Kolodziejczyk A. M., Seto J., Sawyer W. H. Design of more potent antagonists of the antidiuretic responses to arginine-vasopressin. J Med Chem. 1982 Jan;25(1):45–50. doi: 10.1021/jm00343a009. [DOI] [PubMed] [Google Scholar]
- Moos F., Freund-Mercier M. J., Guerné Y., Guerné J. M., Stoeckel M. E., Richard P. Release of oxytocin and vasopressin by magnocellular nuclei in vitro: specific facilitatory effect of oxytocin on its own release. J Endocrinol. 1984 Jul;102(1):63–72. doi: 10.1677/joe.0.1020063. [DOI] [PubMed] [Google Scholar]
- Morris R., Farmery S. M., Roberts C. J., Hill R. G. The effects of oxytocin and vasotocin analogues on the responses of rat brainstem neurones to oxytocin. Neurosci Lett. 1984 Jul 27;48(2):161–166. doi: 10.1016/0304-3940(84)90013-2. [DOI] [PubMed] [Google Scholar]
- Moss R. L., Dyball R. E., Cross B. A. Excitation of antidromically identified neurosecretory cells of the paraventricular nucleus by oxytocin applied iontophoretically. Exp Neurol. 1972 Jan;34(1):95–102. doi: 10.1016/0014-4886(72)90190-2. [DOI] [PubMed] [Google Scholar]
- Mühlethaler M., Dreifuss J. J., Gähwiler B. H. Vasopressin excites hippocampal neurones. Nature. 1982 Apr 22;296(5859):749–751. doi: 10.1038/296749a0. [DOI] [PubMed] [Google Scholar]
- Mühlethaler M., Sawyer W. H., Manning M. M., Dreifuss J. J. Characterization of a uterine-type oxytocin receptor in the rat hippocampus. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6713–6717. doi: 10.1073/pnas.80.21.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Barker J. L. The pharmacology of recurrent inhibition in the supraoptic neurosecretory system. Brain Res. 1971 Dec 24;35(2):501–511. doi: 10.1016/0006-8993(71)90491-4. [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V., Glasmann W. Golgi-like immunoperoxidase staining of hypothalamic magnocellular neurons that contain vasopressin, oxytocin or neurophysin in the rat. Neuroscience. 1981;6(4):619–643. doi: 10.1016/0306-4522(81)90147-0. [DOI] [PubMed] [Google Scholar]
- Suzue T., Yanaihara N., Otsuka M. Actions of vasopressin, gastrin releasing peptide and other peptides on neurons on newborn rat spinal cord in vitro. Neurosci Lett. 1981 Oct 23;26(2):137–142. doi: 10.1016/0304-3940(81)90339-6. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Kuypers H. G. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980 Dec 1;194(3):555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980 Dec;31(6):410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen F. W., Wolters P. Light microscopic autoradiographic localization of [3H]arginine-vasopressin binding sites in the rat brain and kidney. Neurosci Lett. 1983 Oct 31;41(1-2):61–66. doi: 10.1016/0304-3940(83)90223-9. [DOI] [PubMed] [Google Scholar]
- Yamashita H., Inenaga K., Kawata M., Sano Y. Phasically firing neurons in the supraoptic nucleus of the rat hypothalamus: immunocytochemical and electrophysiological studies. Neurosci Lett. 1983 May 27;37(1):87–92. doi: 10.1016/0304-3940(83)90509-8. [DOI] [PubMed] [Google Scholar]


