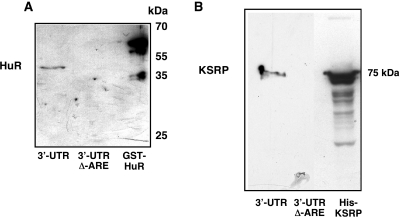
Figure 1.
Purification of RNA-bps interacting with the 3′-UTR of the human iNOS mRNA. To purify proteins binding to the 3′-UTR of the human iNOS mRNA affinity chromatographies using biotinylated iNOS 3′-UTR RNA were performed (45,46) as described in Materials and Methods. DLD-1 cells were preincubated for 18 h in medium without FCS and phenol red. Then cells were incubated with the cytokine mixture for 6 h and protein extracts were isolated. These extracts were incubated with biotinylated iNOS 3′-UTR RNA (3′-UTR) or biotinylated iNOS 3′-UTR RNA without the ARE-sequences (3′-UTR Δ–ARE) and streptavidine-agarose beads. After several washing and centrifugation steps the RNA-bps were eluted by 2 M KCl. (A) Proofing the applicability of this method western blots using a specific anti-HuR antibody were performed, since HuR is known to interact with the human iNOS 3′-UTR (28). As a positive control bacterial expressed GST-HuR fusion protein was also loaded on the SDS gel. One representative blot of three different experiments were shown. (B) The eluates were tested for the presence of KSRP by western blots using a specific anti-KSRP antibody. As a positive control bacterial expressed His-KSRP fusion protein was also loaded on the SDS gel. One representative blot of three different experiments were shown.