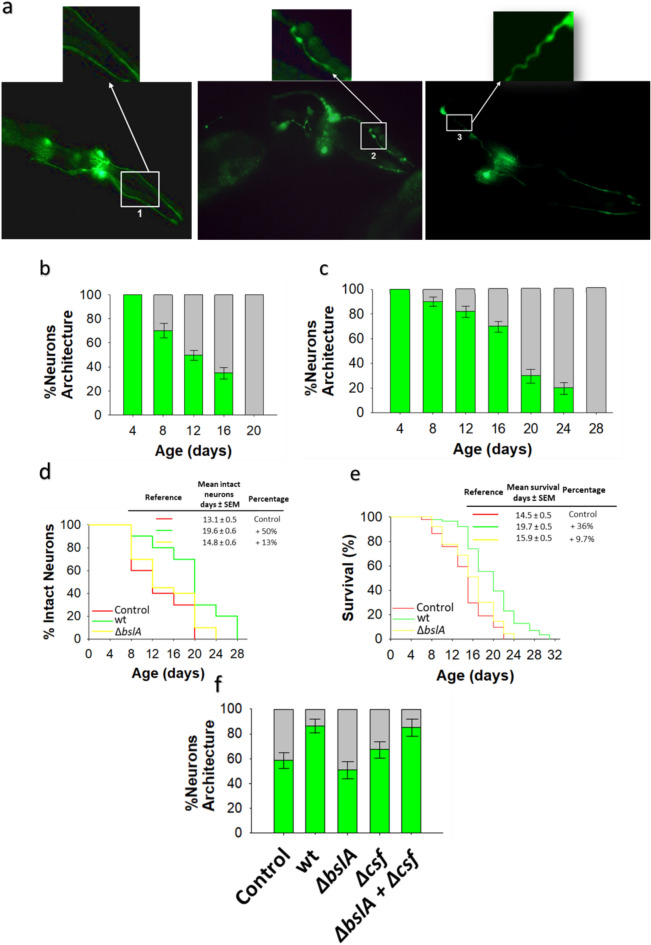
Fig. 3.
Age-related dopaminergic neuron degeneration is delayed by biofilm-forming B. subtilis. (a) Fluorescence images of GFP-tagged dopaminergic neurons of the head region of C. elegans of different ages. Pictures show the neuronal architecture of dopaminergic neurons (CEP and ADE) of E. coli OP50-colonized BZ555 worms of 5 days (left image) and 12 days (middle and right images) of age. Normal neuronal architecture (left); damaged neuronal architecture: blebbing neurite (middle), and wavy neurite (right). (b,c) The degeneration of dopaminergic neurons in the head region of BZ555 worms colonized by E. coli OP50 (b) or biofilm-forming B. subtilis (c). Neuronal degeneration was assessed by semiquantification of the expression of GFP under the control of the dat-1 promotor in the four CEP and two ADE dopaminergic neurons in worms of different ages using a confocal scanning microscope. The percentages of worms with normal neuronal integrity (i.e., worms with absence of dopaminergic neuronal damage, green) and worms presenting dopaminergic neuronal damage (gray) are indicated. See the Methods section for details. (d,e) The percentage of E. coli OP50 (red)-, biofilm-forming B. subtilis (green)-, ΔbslA B. subtilis (yellow)-colonized BZ555 worms with intact neuronal architecture over time and the lifespan of these worms are showed (d,e, respectively). (f) Semiquantification of dopaminergic neurodegeneration in 8-day-old BZ555 worms colonized by E. coli OP50, biofilm-forming B. subtilis or its isogenic mutants ΔbslA, Δcsf, or equal amounts of ΔbslA and Δcsf cells. Semiquantitative dopaminergic neurodegeneration data are presented as the average of three biological replicates, and the error bars represent the standard errors of the mean. Experiments were done by triplicate using 90 animals in each experiment.