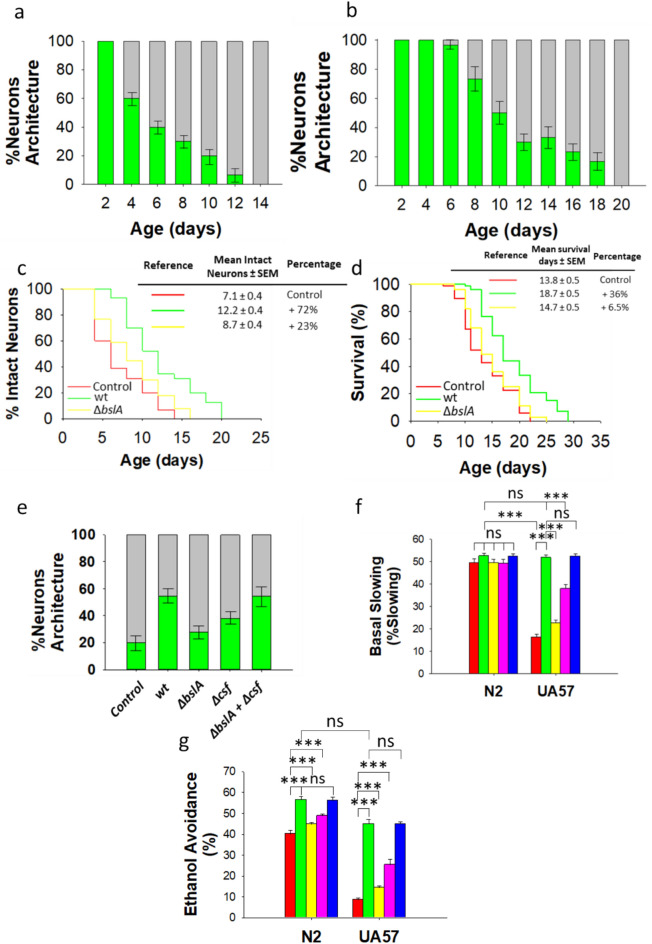
Fig. 4.
Biofilm-proficient B. subtilis delays early dopaminergic neurodegeneration and improves dopamine-dependent behavior in C. elegans. (a,b) The degeneration of dopaminergic neurons in the head region of UA57 worms colonized by E. coli OP50 (a) or biofilm-forming B. subtilis (b). Neuronal degeneration was monitored by semiquantification of the expression of GFP-tagged CEP and ADE dopaminergic neurons. The percentages of worms with normal neuronal integrity (i.e., worms with absence of dopaminergic neuronal damage, green) and worms presenting dopaminergic neuronal damage (gray) are indicated. See the Methods section for details. (c,d) The percentage of E. coli OP50 (red)-, biofilm-forming B. subtilis (green)-, or ΔbslA B. subtilis (yellow)-colonized UA57 worms exhibiting intact neurons (no loss) over time and the lifespan of these worms (c,d, respectively). (e) Semiquantification of dopaminergic neurodegeneration in 8-day-old UA57 worms colonized by E. coli OP50, biofilm-forming B. subtilis or its isogenic mutants ΔbslA, Δcsf, or equal amounts of ΔbslA and Δcsf cells. Semiquantification dopaminergic neurodegeneration data are presented as the average of three biological replicates, and the error bars represent the standard errors of the mean. (f,g) The basal slowing response (f), and the ethanol avoidance (g) in 5-day-old N2 and UA57 worms colonized by E. coli OP50 (red), biofilm-proficient B. subtilis (green), ΔbslA B. subtilis (yellow), Δcsf B. subtilis (pink), or equal amounts of ΔbslA and Δcsf cells (blue) are showed. Each graph (f,g) shows the average ± s.e.m. (n = 3); ns, not significant; p > 0.05; *p < 0.05; ***p < 0.001 (ANOVA followed by Bonferroni’s test). Experiments were done by triplicate using 90 animals in each experiment.