Abstract
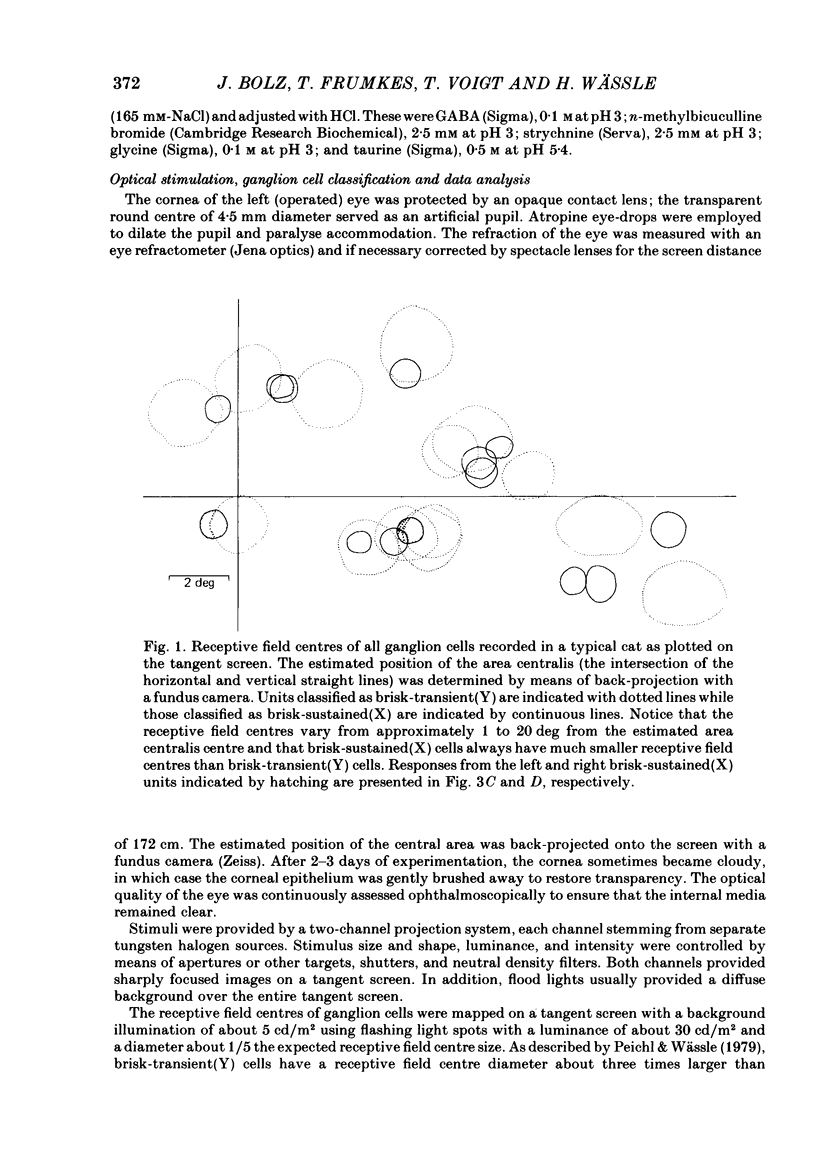
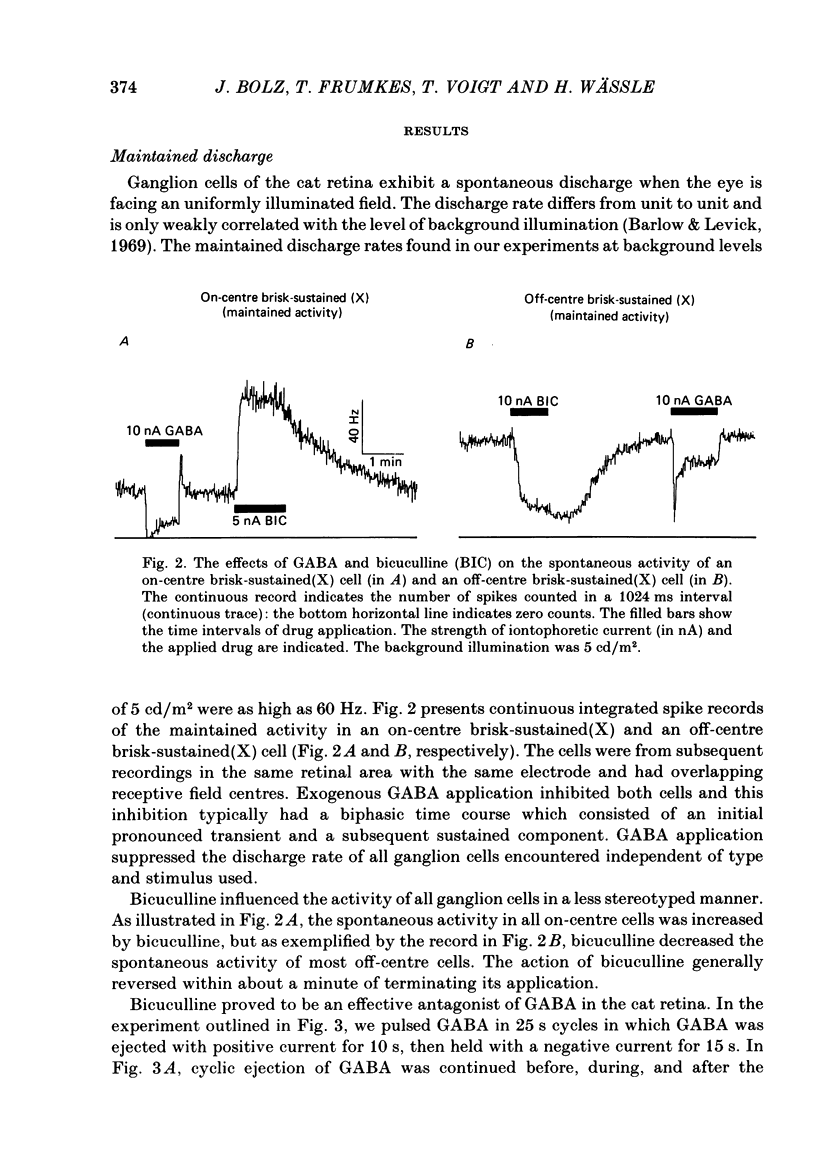
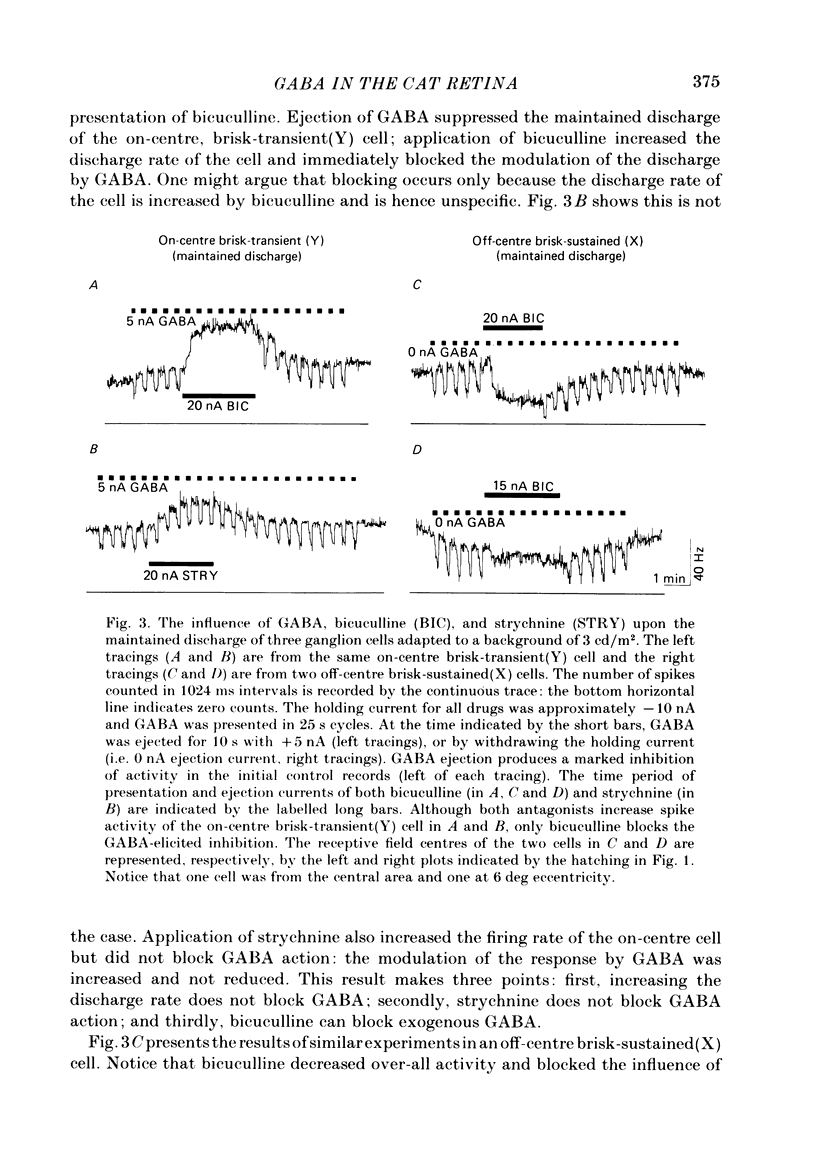
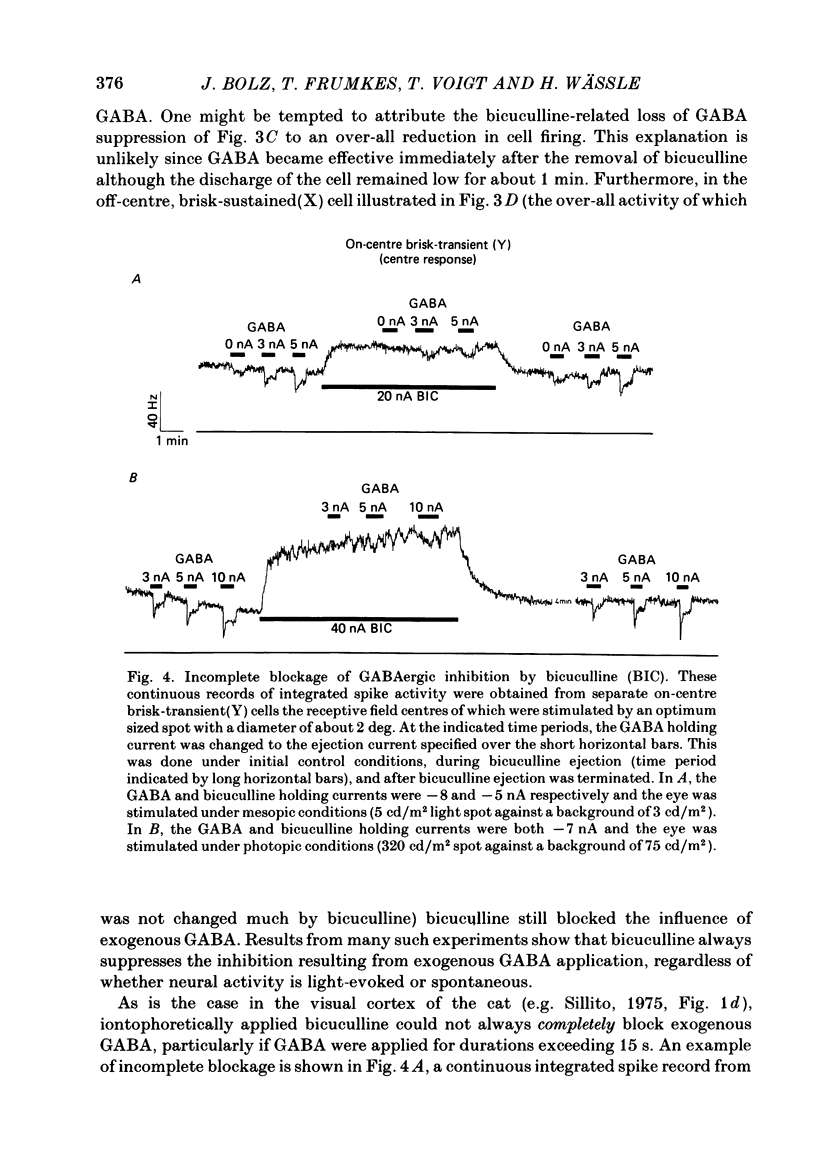
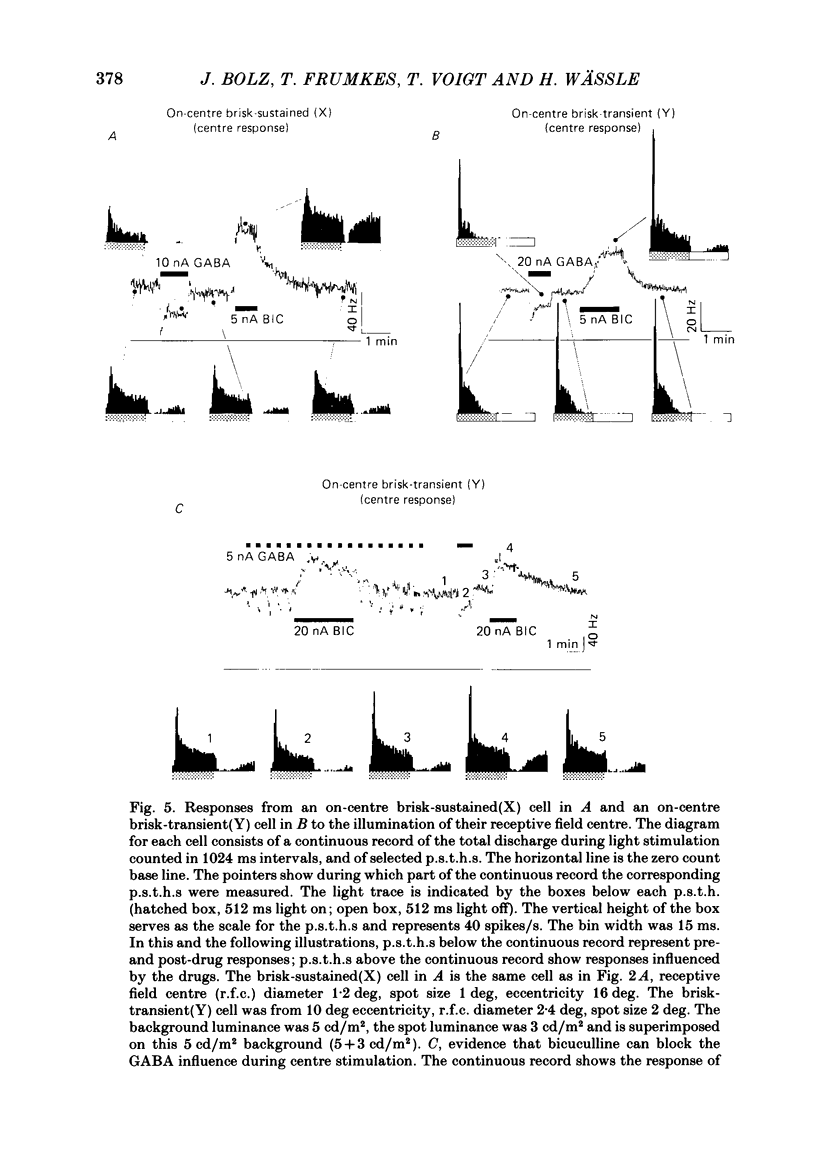
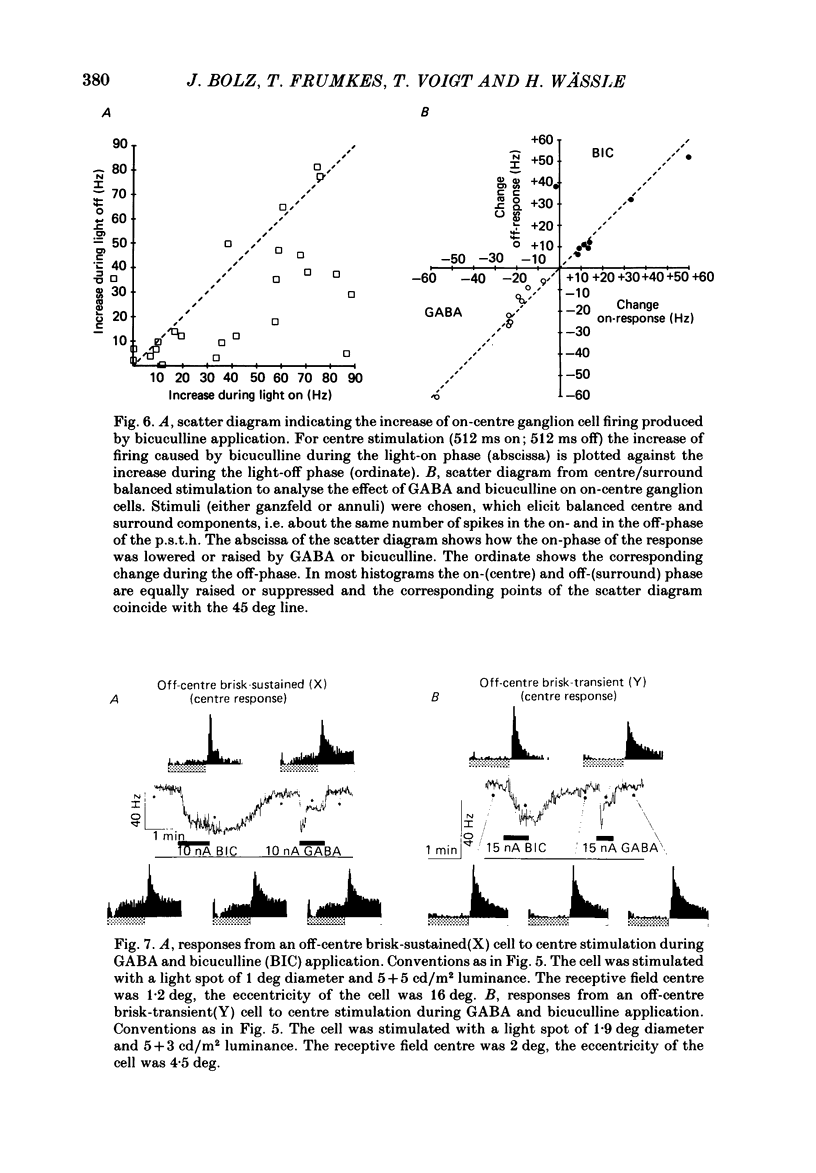
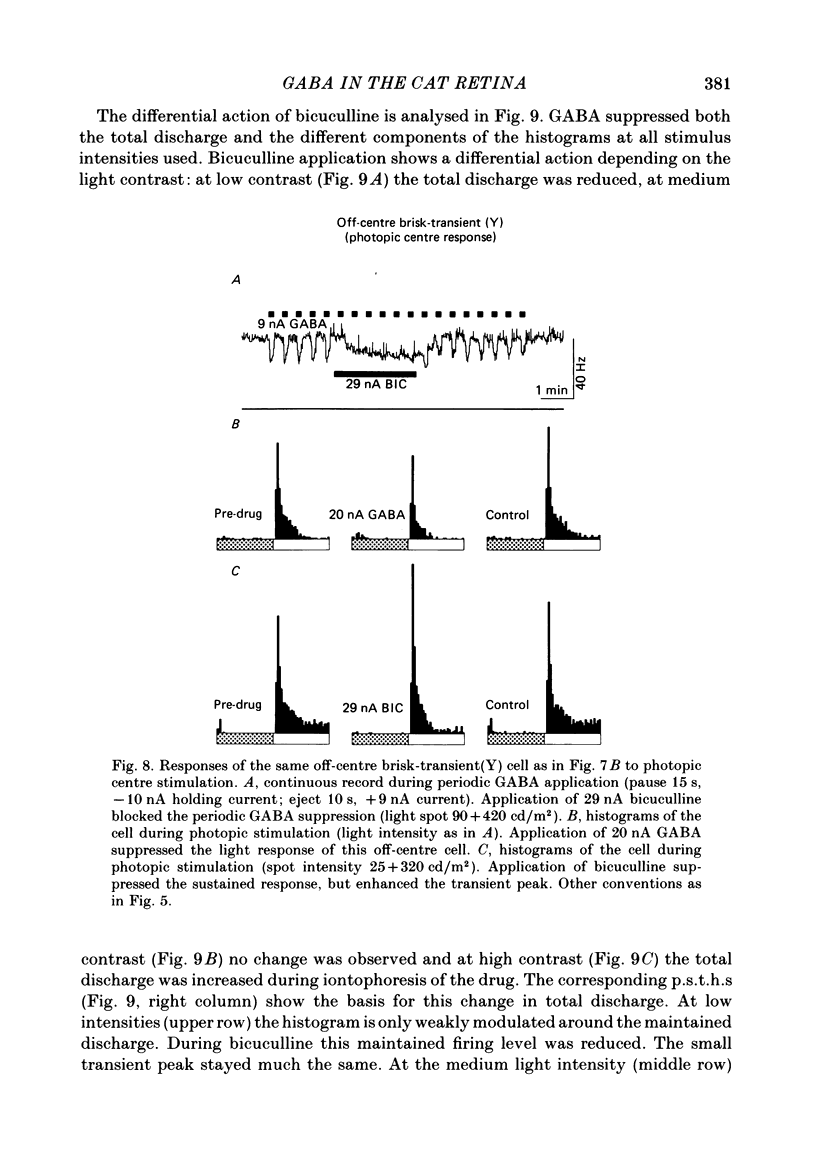
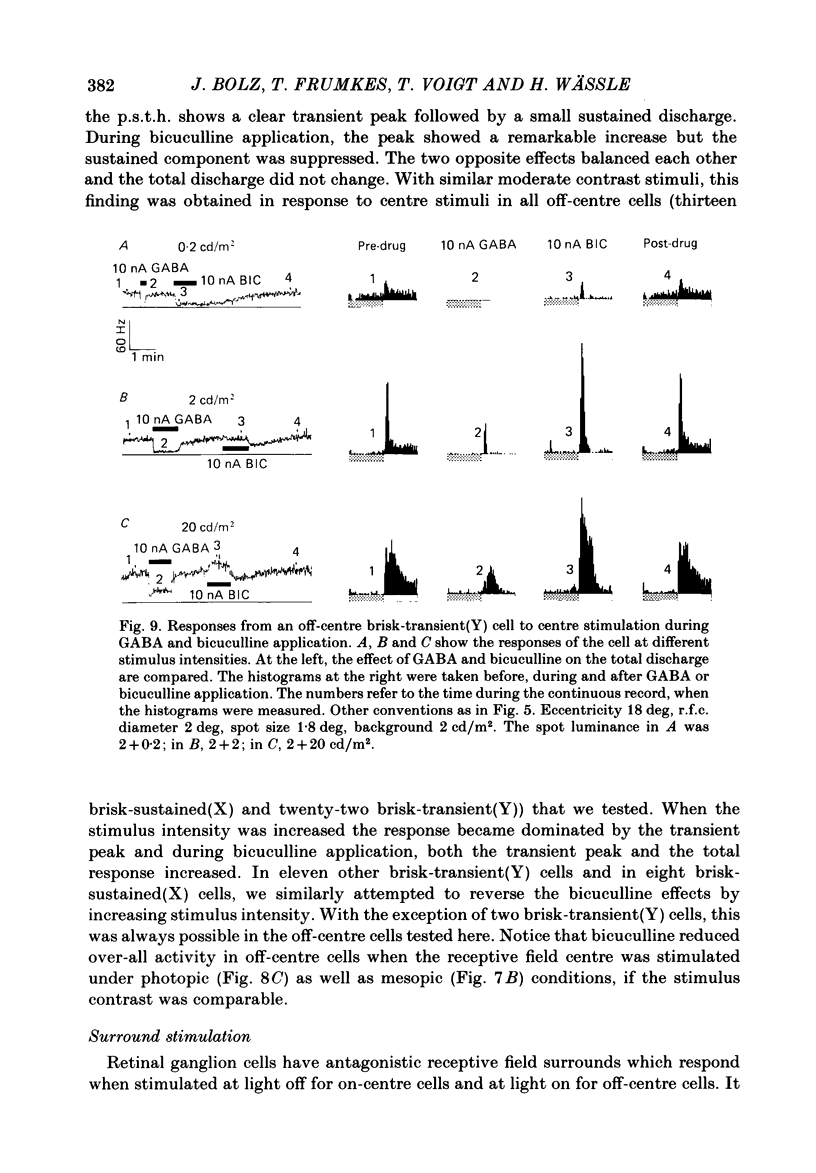
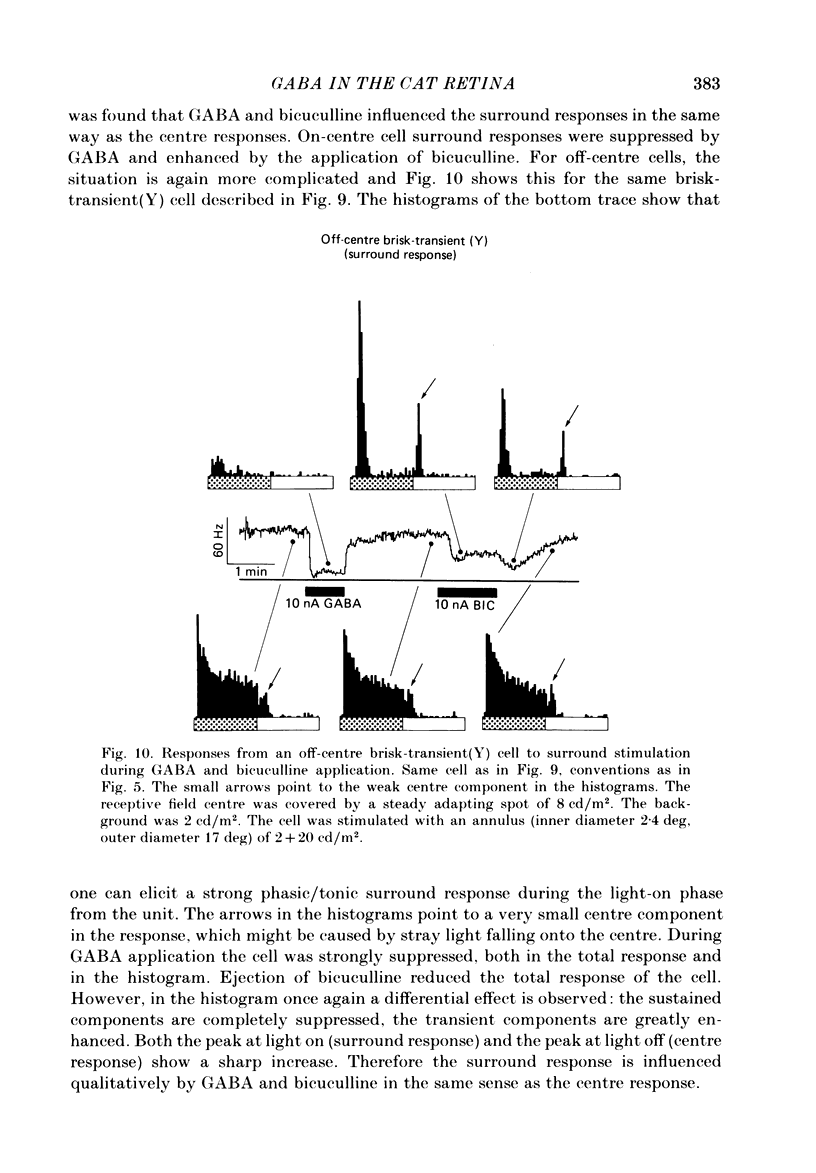
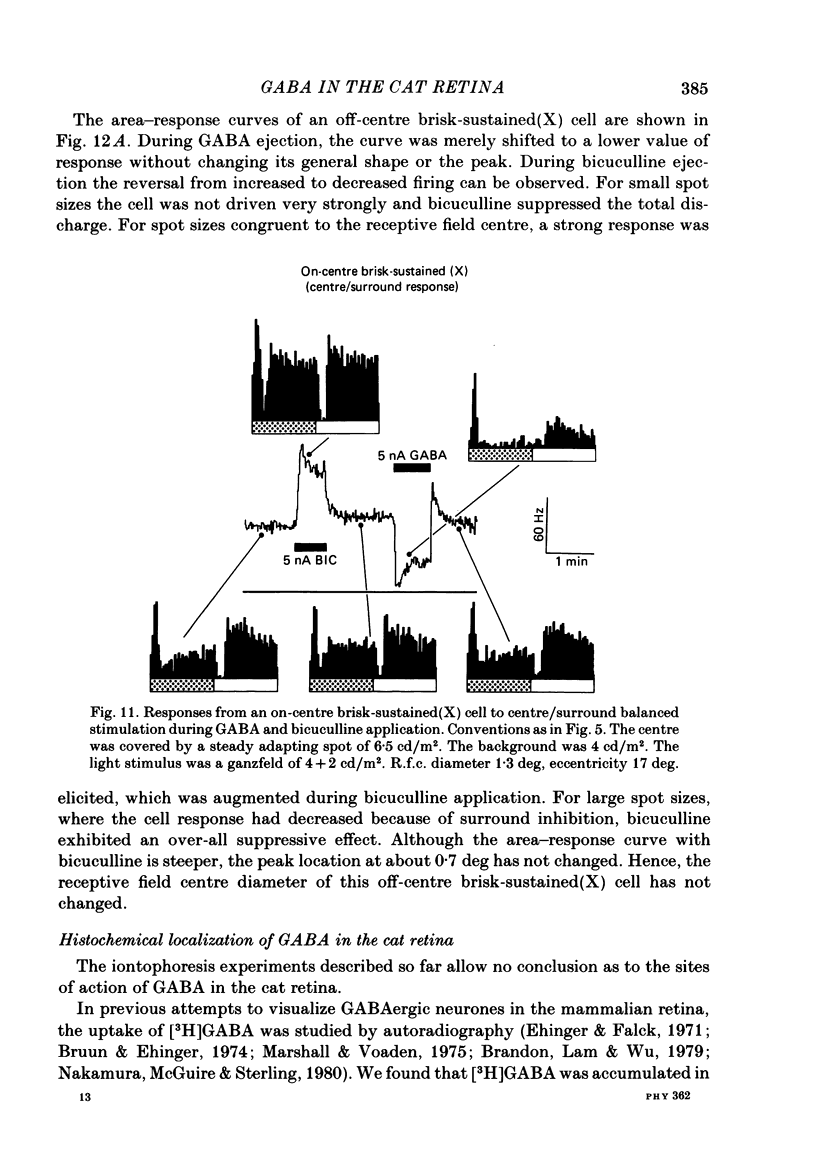
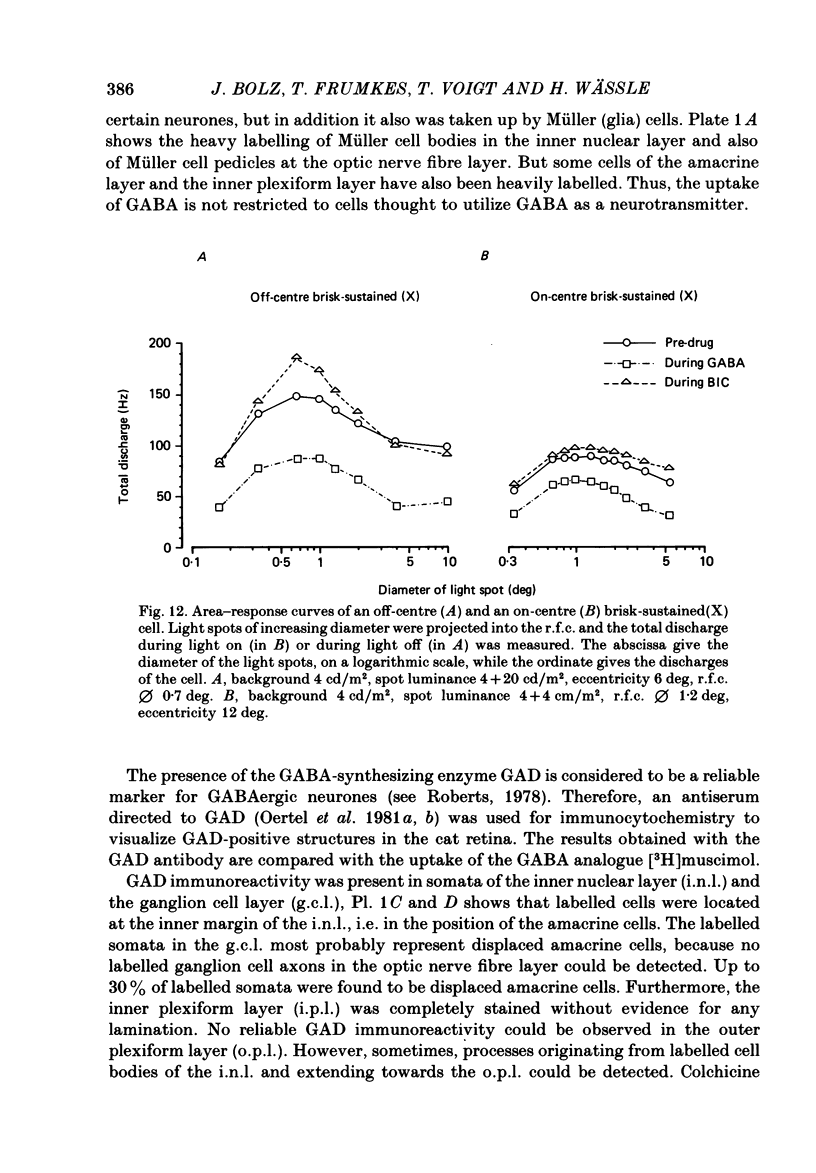
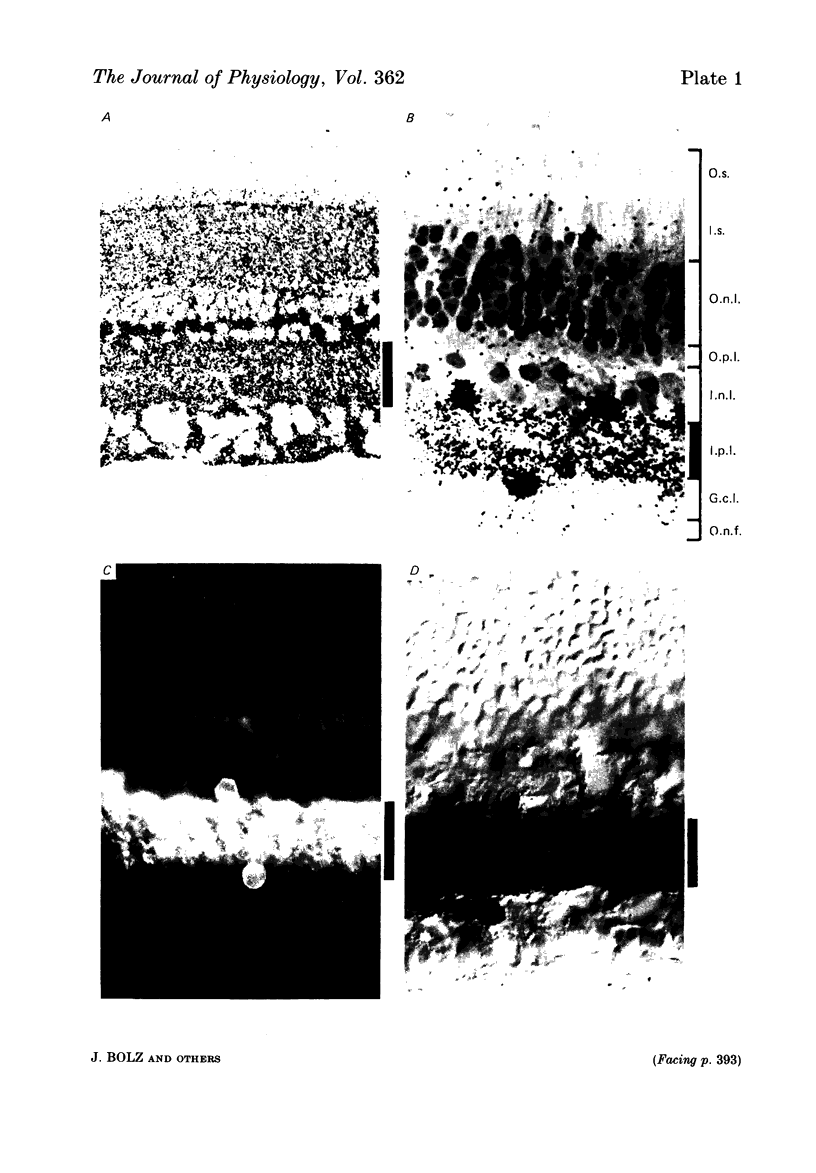
The effects of iontophoretically applied GABA (gamma-aminobutyric acid) and bicuculline on retinal ganglion cells were studied in the optically intact eye of the anaesthetized cat. GABA suppressed both the spontaneous activity and light-evoked discharge of all retinal ganglion cells, regardless of their type and regardless of the visual stimulus used. Bicuculline antagonized the action of iontophoretically applied GABA. Bicuculline enhanced the spontaneous activity of on-centre cells, but suppressed the spontaneous activity of most off-centre cells. The light-evoked response of on-centre cells was increased by bicuculline. A more complicated picture emerged for off-centre cells. Weak light responses were suppressed by bicuculline, but during strong light responses the initial transient phase of the response was dramatically enhanced. Amacrine cells of the inner nuclear layer and displaced amacrine cells of the ganglion cell layer were labelled, using glutamic acid decarboxylase (GAD) immunohistochemistry and [3H]muscimol uptake. GAD-positive dendrites were found throughout the inner plexiform layer and no sign of dendritic stratification was detected.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman R. W., Bader C. R. Biochemical characterization and cellular localization of the cholinergic system in the chicken retina. Brain Res. 1977 Dec 23;138(3):469–485. doi: 10.1016/0006-8993(77)90684-9. [DOI] [PubMed] [Google Scholar]
- Belgum J. H., Dvorak D. R., McReynolds J. S. Sustained synaptic input to ganglion cells of mudpuppy retina. J Physiol. 1982 May;326:91–108. doi: 10.1113/jphysiol.1982.sp014179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz J., Rosner G., Wässle H. Response latency of brisk-sustained (X) and brisk-transient (Y) cells in the cat retina. J Physiol. 1982 Jul;328:171–190. doi: 10.1113/jphysiol.1982.sp014258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz J., Thier P., Voigt T., Wässle H. Action and localization of glycine and taurine in the cat retina. J Physiol. 1985 May;362:395–413. doi: 10.1113/jphysiol.1985.sp015685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz J., Wässle H., Thier P. Pharmacological modulation of on and off ganglion cells in the cat retina. Neuroscience. 1984 Jul;12(3):875–885. doi: 10.1016/0306-4522(84)90176-3. [DOI] [PubMed] [Google Scholar]
- Brandon C., Lam D. M., Wu J. Y. The gamma-aminobutyric acid system in rabbit retina: localization by immunocytochemistry and autoradiography. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3557–3561. doi: 10.1073/pnas.76.7.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruun A., Ehinger B. Uptake of certain possible neurotransmitters into retinal neurons of some mammals. Exp Eye Res. 1974 Nov;19(5):435–447. doi: 10.1016/0014-4835(74)90052-9. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Brisk and sluggish concentrically organized ganglion cells in the cat's retina. J Physiol. 1974 Jul;240(2):421–456. doi: 10.1113/jphysiol.1974.sp010617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Properties of rarely encountered types of ganglion cells in the cat's retina and an overall classification. J Physiol. 1974 Jul;240(2):457–492. doi: 10.1113/jphysiol.1974.sp010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Ariel M., Caldwell J. H. Function of neurotransmitters in the retina. Retina. 1982;2(4):322–331. [PubMed] [Google Scholar]
- Ehinger B., Falck B. Autoradiography of some suspected neurotransmitter substances: GABA glycine, glutamic acid, histamine, dopamine, and L-dopa. Brain Res. 1971 Oct 8;33(1):157–172. doi: 10.1016/0006-8993(71)90314-3. [DOI] [PubMed] [Google Scholar]
- Ehinger B. Neurotransmitter systems in the retina. Retina. 1982;2(4):305–321. [PubMed] [Google Scholar]
- Famiglietti E. V., Jr, Vaughn J. E. Golgi-impregnated amacrine cells and GABAergic retinal neurons: a comparison of dendritic, immunocytochemical and histochemical stratification in the inner plexiform layer of rat retina. J Comp Neurol. 1981 Mar 20;197(1):129–139. doi: 10.1002/cne.901970110. [DOI] [PubMed] [Google Scholar]
- Freed M. A., Nakamura Y., Sterling P. Four types of amacrine in the cat retina that accumulate GABA. J Comp Neurol. 1983 Sep 20;219(3):295–304. doi: 10.1002/cne.902190305. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Sheardown M. J. Transmitters mediating inhibition of ganglion cells in the cat retina: iontophoretic studies in vivo. Neuroscience. 1983 Apr;8(4):837–853. doi: 10.1016/0306-4522(83)90014-3. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Mitchell J. F., Srinivasan V. The release of gamma-aminobutyric acid during inhibition in the cat visual cortex. J Physiol. 1971 Jan;212(2):519–534. doi: 10.1113/jphysiol.1971.sp009339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Karten H. J., Brecha N. Localization of neuroactive substances in the vertebrate retina: evidence for lamination in the inner plexiform layer. Vision Res. 1983;23(10):1197–1205. doi: 10.1016/0042-6989(83)90033-0. [DOI] [PubMed] [Google Scholar]
- Kirby A. W., Enroth-Cugell C. The involvement of gamma-aminobutyric acid in the organization of cat retinal ganglion cell receptive fields. A study with picrotoxin and bicuculline. J Gen Physiol. 1976 Oct;68(4):465–484. doi: 10.1085/jgp.68.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby A. W., Schweitzer-Tong D. E. GABA-antagonists and spatial summation in Y-type cat retinal ganglion cells. J Physiol. 1981 Mar;312:335–344. doi: 10.1113/jphysiol.1981.sp013631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby A. W. The effect of strychnine, bicuculline, and picrotoxin on X and Y cells in the cat retina. J Gen Physiol. 1979 Jul;74(1):71–84. doi: 10.1085/jgp.74.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. The inner plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol. 1979 Jun;8(3):295–329. doi: 10.1007/BF01236124. [DOI] [PubMed] [Google Scholar]
- Lam D. M. Biosynthesis of gamma-aminobutyric acid by isolated axons of cone horizontal cells in the goldfish retina. Nature. 1975 Mar 27;254(5498):345–347. doi: 10.1038/254345a0. [DOI] [PubMed] [Google Scholar]
- Lam D. M., Su Y. Y., Swain L., Marc R. E., Brandon C., Wu J. Y. Immunocytochemical localisation of L-glutamic acid decarboxylase in the goldfish retina. Nature. 1979 Apr 5;278(5704):565–567. doi: 10.1038/278565a0. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Marshall J., Voaden M. Autoradiographic identification of the cells accumulating 3H gamma-aminobutyric acid in mammalian retinae: a species comparison. Vision Res. 1975 Mar;15(3):459–461. doi: 10.1016/0042-6989(75)90102-9. [DOI] [PubMed] [Google Scholar]
- Masland R. H., Mills J. W. Autoradiographic identification of acetylcholine in the rabbit retina. J Cell Biol. 1979 Oct;83(1):159–178. doi: 10.1083/jcb.83.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Miller R. F., Dacheux R. F., Frumkes T. E. Amacrine cells in Necturus retina: evidence for independent gamma-aminobutyric acid- and glycine-releasing neurons. Science. 1977 Nov 18;198(4318):748–750. doi: 10.1126/science.910159. [DOI] [PubMed] [Google Scholar]
- Miller R. F., Frumkes T. E., Slaughter M., Dacheux R. F. Physiological and pharmacological basis of GABA and glycine action on neurons of mudpuppy retina. I. Receptors, horizontal cells, bipolars, and G-cells. J Neurophysiol. 1981 Apr;45(4):743–763. doi: 10.1152/jn.1981.45.4.743. [DOI] [PubMed] [Google Scholar]
- Miller R. F., Frumkes T. E., Slaughter M., Dacheux R. F. Physiological and pharmacological basis of GABA and glycine action on neurons of mudpuppy retina. I. Receptors, horizontal cells, bipolars, and G-cells. J Neurophysiol. 1981 Apr;45(4):743–763. doi: 10.1152/jn.1981.45.4.743. [DOI] [PubMed] [Google Scholar]
- Miller R. F., Frumkes T. E., Slaughter M., Dacheux R. F. Physiological and pharmacological basis of GABA and glycine action on neurons of mudpuppy retina. II. Amacrine and ganglion cells. J Neurophysiol. 1981 Apr;45(4):764–782. doi: 10.1152/jn.1981.45.4.764. [DOI] [PubMed] [Google Scholar]
- Murakami M., Shimoda Y., Nakatani K., Miyachi E., Watanabe S. GABA-mediated negative feedback and color opponency in carp retina. Jpn J Physiol. 1982;32(6):927–935. doi: 10.2170/jjphysiol.32.927. [DOI] [PubMed] [Google Scholar]
- Murakami M., Shimoda Y., Nakatani K., Miyachi E., Watanabe S. GABA-mediated negative feedback from horizontal cells to cones in carp retina. Jpn J Physiol. 1982;32(6):911–926. doi: 10.2170/jjphysiol.32.911. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., McGuire B. A., Sterling P. Interplexiform cell in cat retina: identification by uptake of gamma-[3H]aminobutyric acid and serial reconstruction. Proc Natl Acad Sci U S A. 1980 Jan;77(1):658–661. doi: 10.1073/pnas.77.1.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R., Famiglietti E. V., Jr, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978 Mar;41(2):472–483. doi: 10.1152/jn.1978.41.2.472. [DOI] [PubMed] [Google Scholar]
- Oertel W. H., Schmechel D. E., Tappaz M. L., Kopin I. J. Production of a specific antiserum to rat brain glutamic acid decarboxylase by injection of an antigen-antibody complex. Neuroscience. 1981;6(12):2689–2700. doi: 10.1016/0306-4522(81)90113-5. [DOI] [PubMed] [Google Scholar]
- Oertel W. H., Schmechel D. E., Weise V. K., Ransom D. H., Tappaz M. L., Krutzsch H. C., Kopin I. J. Comparison of cysteine sulphinic acid decarboxylase isoenzymes and glutamic acid decarboxylase in rat liver and brain. Neuroscience. 1981;6(12):2701–2714. doi: 10.1016/0306-4522(81)90114-7. [DOI] [PubMed] [Google Scholar]
- Peichl L., Wässle H. Size, scatter and coverage of ganglion cell receptive field centres in the cat retina. J Physiol. 1979 Jun;291:117–141. doi: 10.1113/jphysiol.1979.sp012803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L., Wässle H. The structural correlate of the receptive field centre of alpha ganglion cells in the cat retina. J Physiol. 1983 Aug;341:309–324. doi: 10.1113/jphysiol.1983.sp014807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcho R. G. Autoradiographic localization of [3H]muscimol in the cat retina. Brain Res. 1981 Jun 29;215(1-2):187–199. doi: 10.1016/0006-8993(81)90501-1. [DOI] [PubMed] [Google Scholar]
- Pourcho R. G., Goebel D. J. Neuronal subpopulations in cat retina which accumulate the GABA agonist, (3H)muscimol: a combined Golgi and autoradiographic study. J Comp Neurol. 1983 Sep 1;219(1):25–35. doi: 10.1002/cne.902190104. [DOI] [PubMed] [Google Scholar]
- Saito H. Pharmacological and morphological differences between X- and Y-type ganglion cells in the cat's retina. Vision Res. 1983;23(11):1299–1308. doi: 10.1016/0042-6989(83)90105-0. [DOI] [PubMed] [Google Scholar]
- Saito H. The effects of strychnine and bicuculline on the responses of X- and Y-cells of the isolated eye-cut preparation of the cat. Brain Res. 1981 May 11;212(1):243–248. doi: 10.1016/0006-8993(81)90061-5. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. The effectiveness of bicuculline as an antagonist of GABA and visually evoked inhibition in the cat's striate cortex. J Physiol. 1975 Sep;250(2):287–304. doi: 10.1113/jphysiol.1975.sp011055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P. Microcircuitry of the cat retina. Annu Rev Neurosci. 1983;6:149–185. doi: 10.1146/annurev.ne.06.030183.001053. [DOI] [PubMed] [Google Scholar]
- Straschill M., Perwein J. The inhibition of retinal ganglion cells by catecholeamines and gamma-aminobutyric acid. Pflugers Arch. 1969;312(3):45–54. doi: 10.1007/BF00588530. [DOI] [PubMed] [Google Scholar]
- Thier P., Alder V. Action of iontophoretically applied dopamine on cat retinal ganglion cells. Brain Res. 1984 Jan 30;292(1):109–121. doi: 10.1016/0006-8993(84)90895-3. [DOI] [PubMed] [Google Scholar]
- Thier P., Wässle H. Indoleamine-mediated reciprocal modulation of on-centre and off-centre ganglion cell activity in the retina of the cat. J Physiol. 1984 Jun;351:613–630. doi: 10.1113/jphysiol.1984.sp015266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J. E., Famiglietti E. V., Jr, Barber R. P., Saito K., Roberts E., Ribak C. E. GABAergic amacrine cells in rat retina: immunocytochemical identification and synaptic connectivity. J Comp Neurol. 1981 Mar 20;197(1):113–127. doi: 10.1002/cne.901970109. [DOI] [PubMed] [Google Scholar]



