Abstract
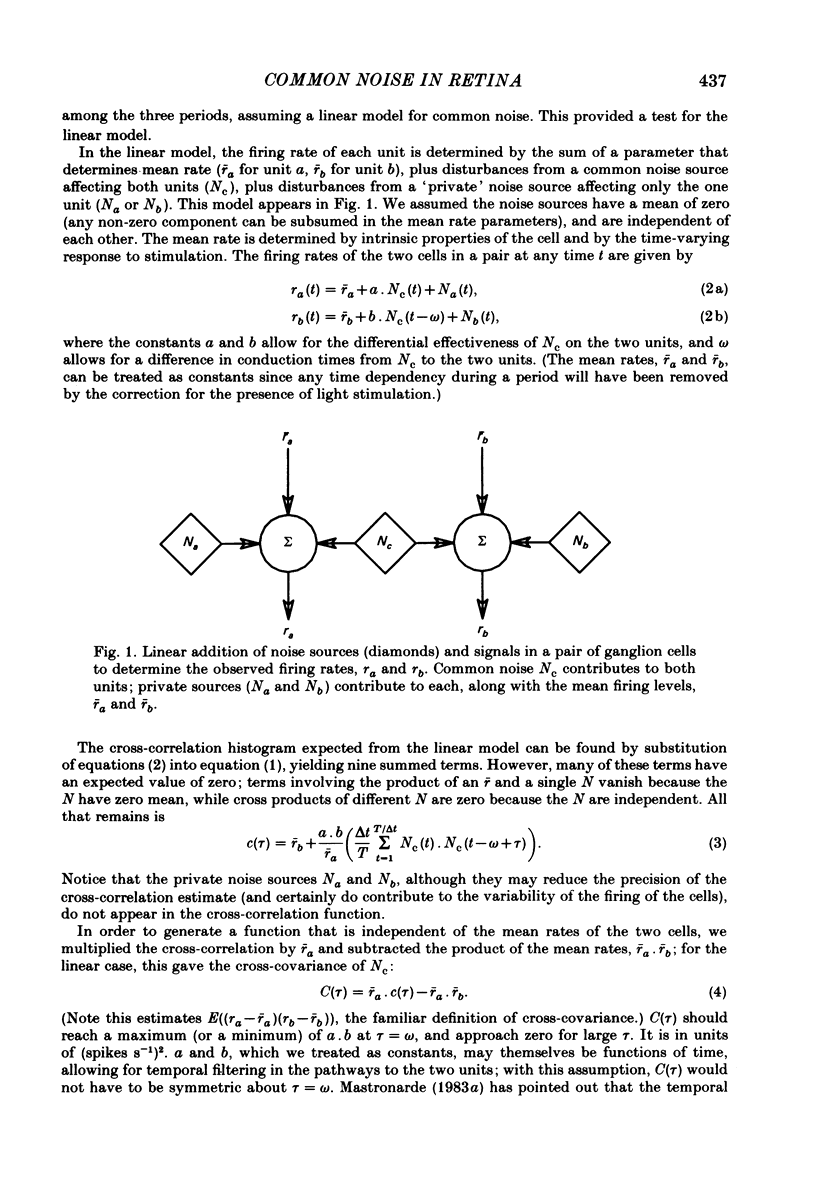
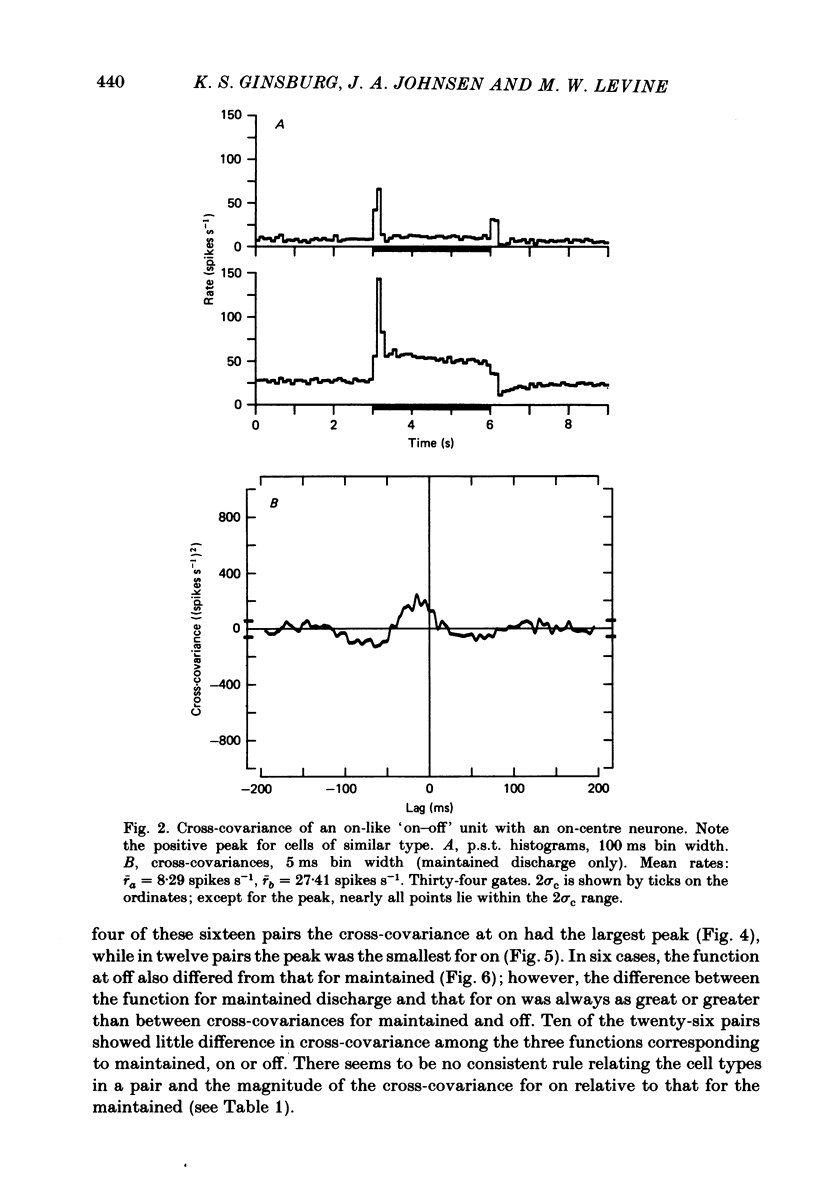
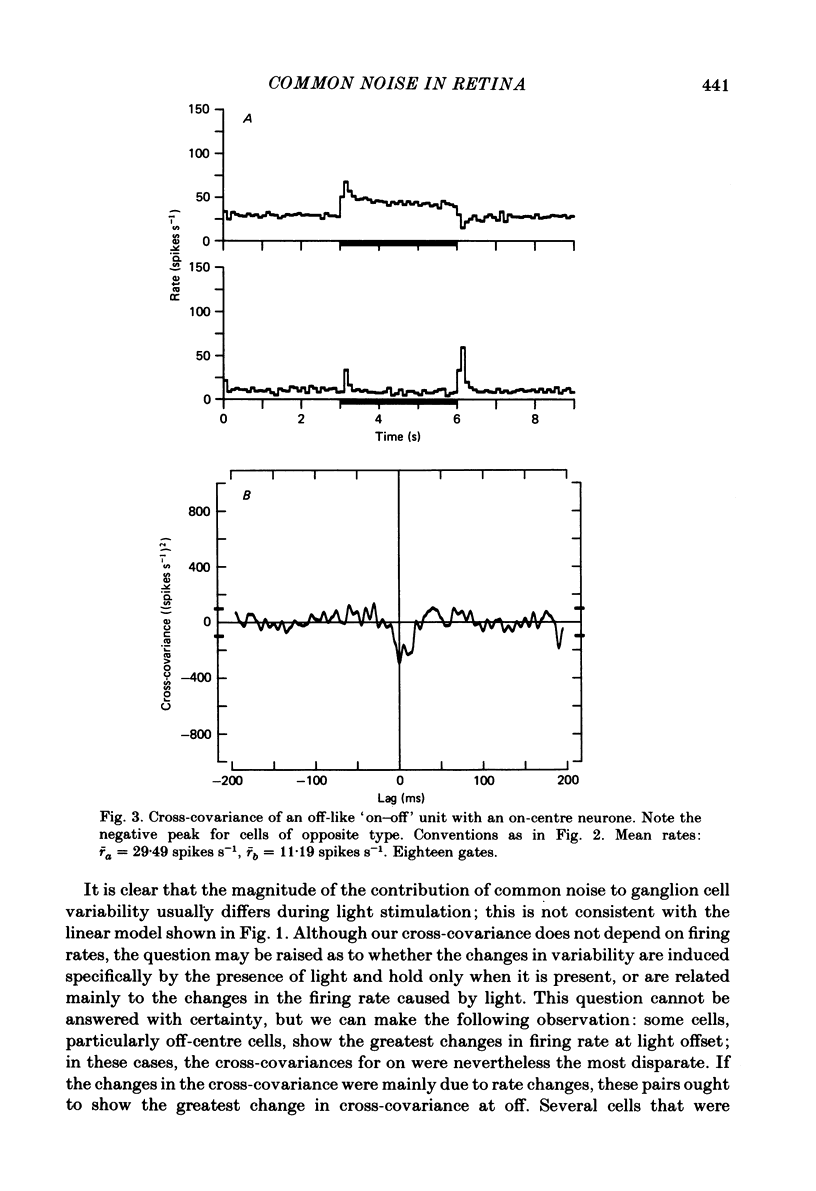
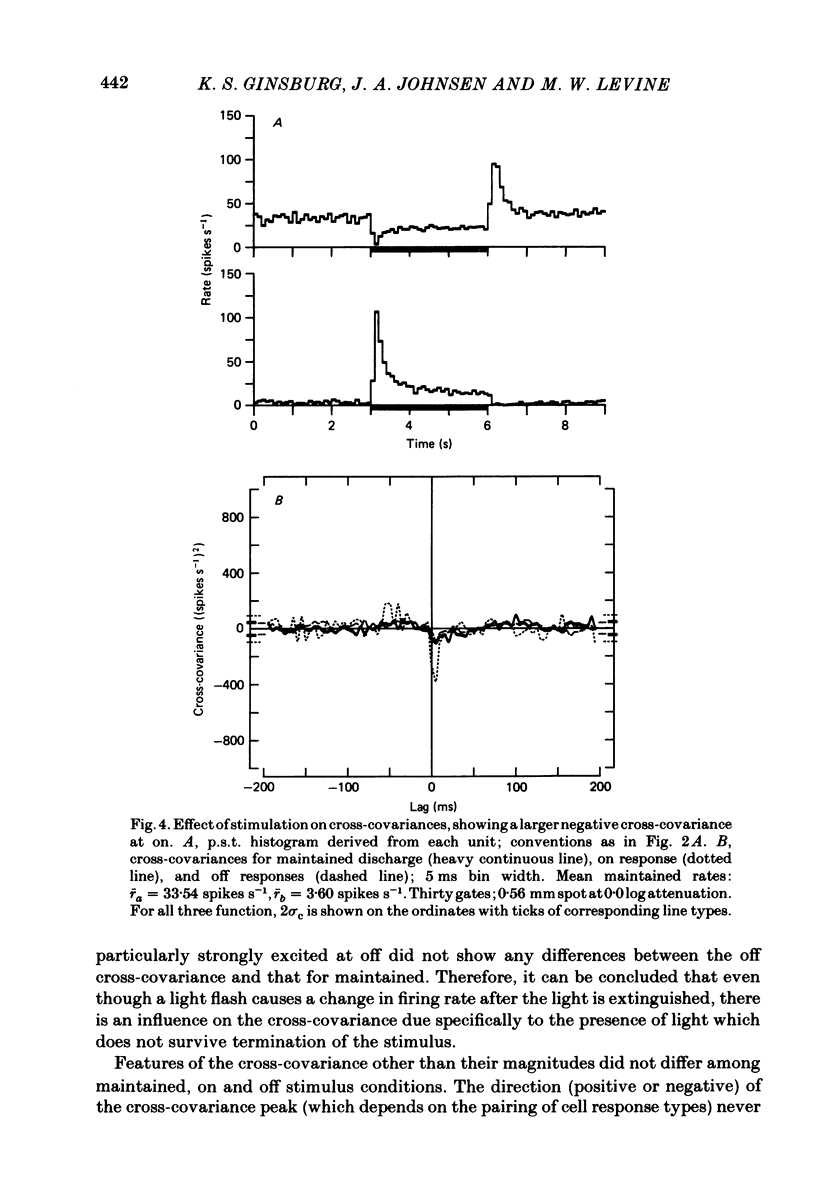
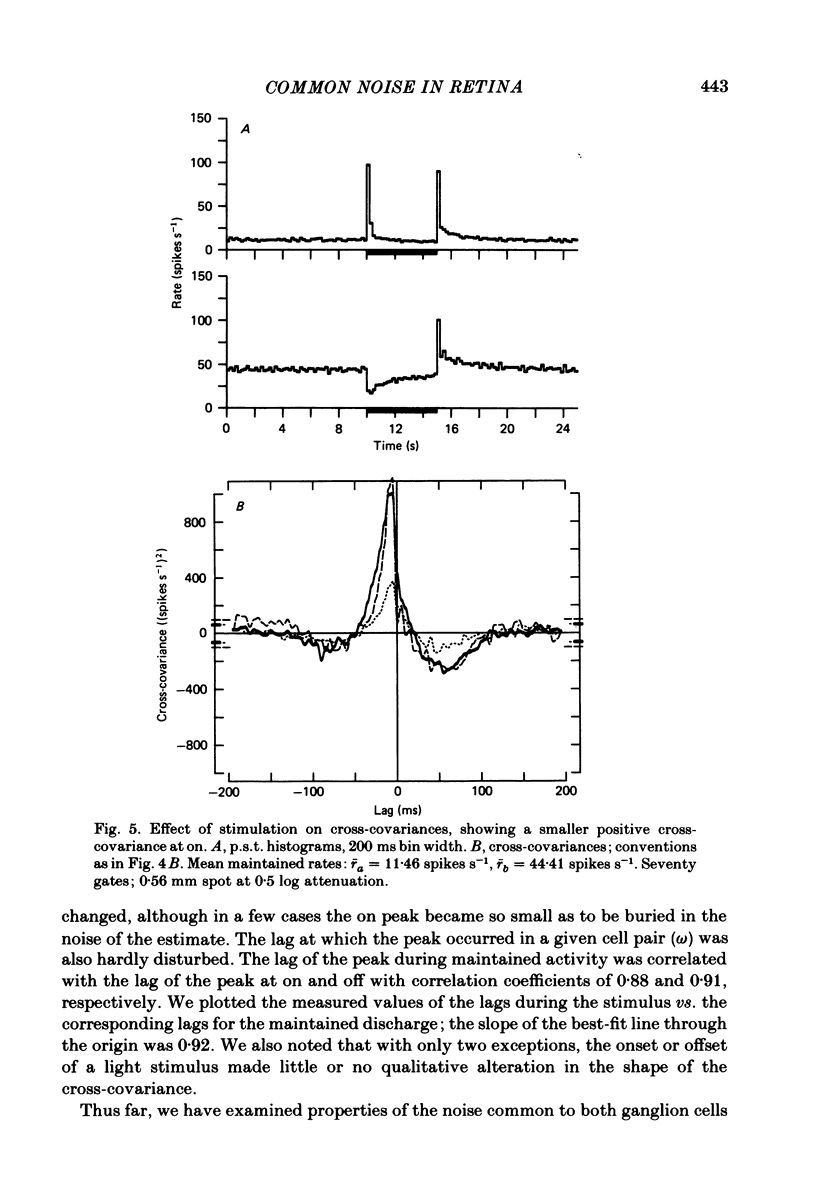
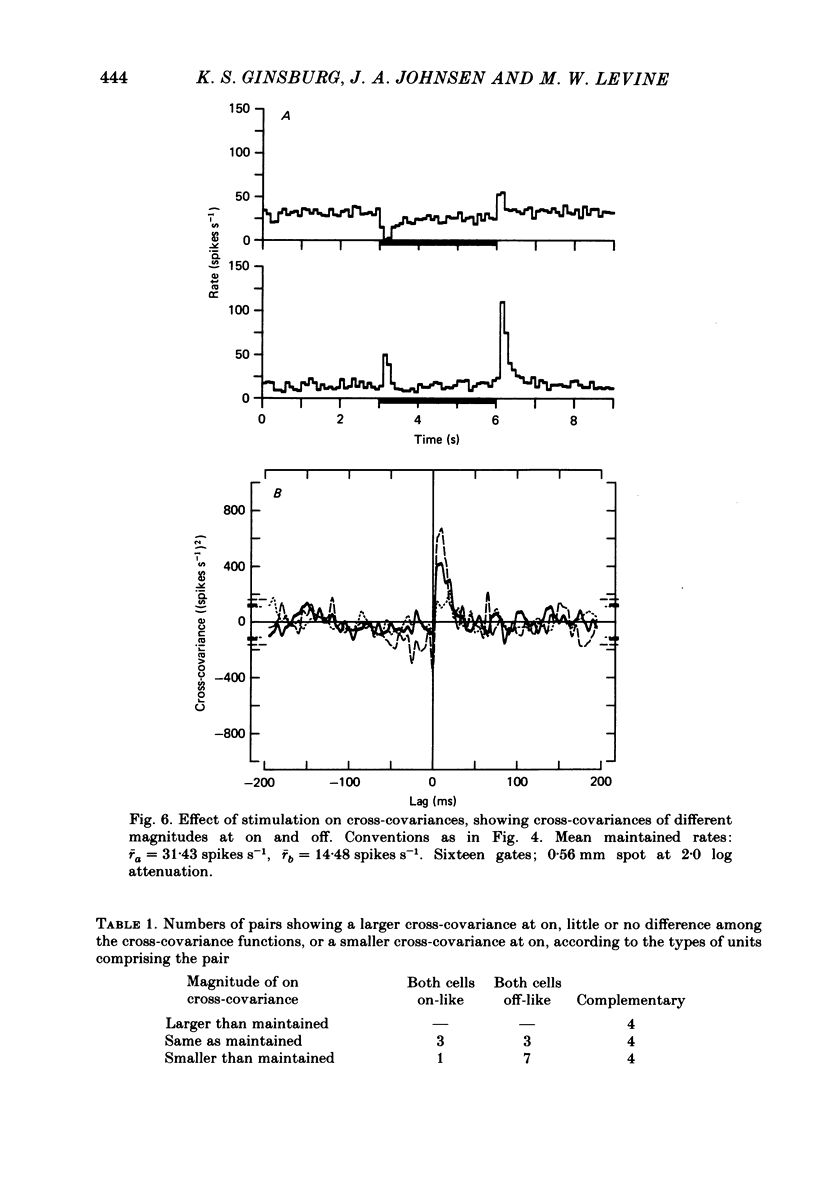
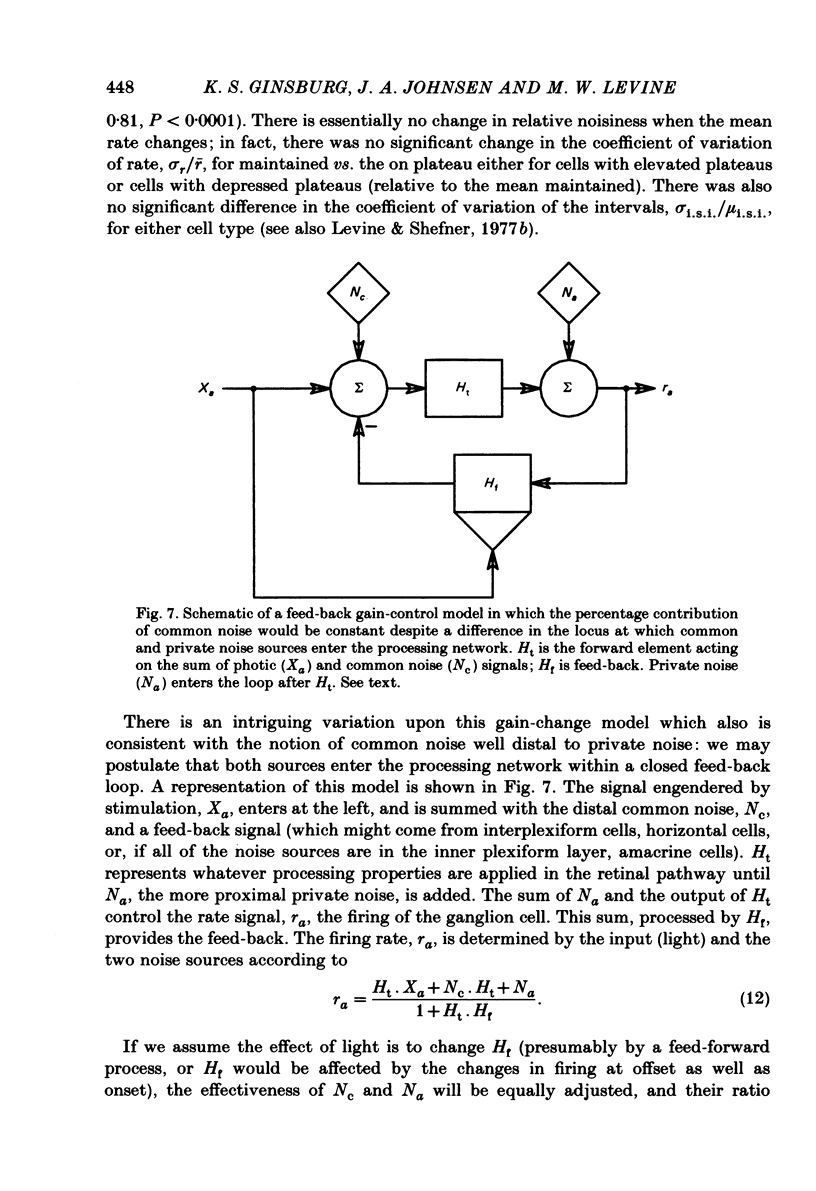
Pairs of goldfish retinal ganglion cells with overlapping receptive fields were recorded during stimulation with repeated light flashes. Cross-correlation histograms for 'maintained' discharge, 'on' responses, and 'off' responses were computed with a correction for the systematic responses to the stimuli; cross-covariances were derived from these. If stimulus-induced signals and noise combine linearly, then the cross-covariances are independent of differences in mean firing rate. Cross-covariances of pairs of cells with the same response polarity displayed a positive peak near zero lag; pairs with complementary responses showed a negative peak. 'On-off' cells could generally be classified as on-like or off-like, based on the plateau of firing during a prolonged flash and the relative magnitudes of the on and off peak responses; the cross-covariances of these cells were as one would predict if they were pure on- or off-centre neurones. The cross-covariances derived from the on period usually differed in magnitude from those derived in the dark (either maintained or off response). In general, cross-covariances for off responses were nearly identical to those for the maintained discharges of the same pair, although the mean rates at off were usually quite different from the maintained. The change in magnitude of the cross-covariances from on responses therefore appears to be a non-linear effect of light, and not of the changes in firing rate induced by the light. Other features of the cross-covariances were not affected by stimulation. The general shapes remained fairly constant, and the lags at which the peaks occurred were not consistently affected. We estimated the variance of the firing rate of each unit in three ways, and used two methods of portioning the variance implied by the cross-covariances; from these estimates, we obtained an upper bound for the proportion of the variance of firing of a cell which is due to the common noise that affects both members of a pair. We found that the common influence accounts for less than 20% of the total variance. During stimulation, both the magnitude of the cross-covariance and the variance of the rates change; however, the percentage of total variance contributed by the common noise source is constant. We conclude that light has the effect of changing the gain of the pathway after the introduction of both the common and unshared (private) noise sources but before the ganglion cell.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett D. W. Statistical dependence between neighboring retinal ganglion cells in goldfish. Exp Brain Res. 1978 May 12;32(1):49–53. doi: 10.1007/BF00237389. [DOI] [PubMed] [Google Scholar]
- Arnett D., Spraker T. E. Cross-correlation analysis of the maintained discharge of rabbit retinal ganglion cells. J Physiol. 1981 Aug;317:29–47. doi: 10.1113/jphysiol.1981.sp013812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W. Colour-coded ganglion cells in the goldfish retina: extension of their receptive fields by means of new stimuli. J Physiol. 1968 Aug;197(3):567–592. doi: 10.1113/jphysiol.1968.sp008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frishman L. J., Levine M. W. Statistics of the maintained discharge of cat retinal ganglion cells. J Physiol. 1983 Jun;339:475–494. doi: 10.1113/jphysiol.1983.sp014728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen J. A., Levine M. W. Correlation of activity in neighbouring goldfish ganglion cells: relationship between latency and lag. J Physiol. 1983 Dec;345:439–449. doi: 10.1113/jphysiol.1983.sp014987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. H., Kiang N. Y. Analysis of discharges recorded simultaneously from pairs of auditory nerve fibers. Biophys J. 1976 Jul;16(7):719–734. doi: 10.1016/S0006-3495(76)85724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Simon E. J. Analysis of electrical noise in turtle cones. J Physiol. 1977 Nov;272(2):435–468. doi: 10.1113/jphysiol.1977.sp012053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. W. Firing rate of a retinal neuron are not predictable from interspike interval statistics. Biophys J. 1980 Apr;30(1):9–25. doi: 10.1016/S0006-3495(80)85073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. W. Retinal processing of intrinsic ad extrinsic noise. J Neurophysiol. 1982 Oct;48(4):992–1010. doi: 10.1152/jn.1982.48.4.992. [DOI] [PubMed] [Google Scholar]
- Levine M. W., Shefner J. M. Independence of "on" and "off" responses of retinal ganglion cells. Science. 1975 Dec 19;190(4220):1215–1217. doi: 10.1126/science.1239079. [DOI] [PubMed] [Google Scholar]
- Levine M. W., Shefner J. M. The effects of photic stimulation upon the variability of the interspike intervals in goldfish ganglion cells. Vision Res. 1977;17(7):793–797. doi: 10.1016/0042-6989(77)90121-3. [DOI] [PubMed] [Google Scholar]
- Levine M. W., Shefner J. M. Variability in ganglion cell firing patterns; implications for separate "on" and "off" processes. Vision Res. 1977;17(7):765–776. doi: 10.1016/0042-6989(77)90118-3. [DOI] [PubMed] [Google Scholar]
- MACNICHOL E. J., SVAETICHIN G. Electric responses from the isolated retinas of fishes. Am J Ophthalmol. 1958 Sep;46(3 Pt 2):26–46. doi: 10.1016/0002-9394(58)90053-9. [DOI] [PubMed] [Google Scholar]
- Mastronarde D. N. Correlated firing of cat retinal ganglion cells. I. Spontaneously active inputs to X- and Y-cells. J Neurophysiol. 1983 Feb;49(2):303–324. doi: 10.1152/jn.1983.49.2.303. [DOI] [PubMed] [Google Scholar]
- Mastronarde D. N. Correlated firing of cat retinal ganglion cells. II. Responses of X- and Y-cells to single quantal events. J Neurophysiol. 1983 Feb;49(2):325–349. doi: 10.1152/jn.1983.49.2.325. [DOI] [PubMed] [Google Scholar]
- Mastronarde D. N. Interactions between ganglion cells in cat retina. J Neurophysiol. 1983 Feb;49(2):350–365. doi: 10.1152/jn.1983.49.2.350. [DOI] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. II. Simultaneous spike trains. Biophys J. 1967 Jul;7(4):419–440. doi: 10.1016/S0006-3495(67)86597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck R. W. Maintained activity of cat retinal ganglion cells. J Neurophysiol. 1967 Sep;30(5):1043–1071. doi: 10.1152/jn.1967.30.5.1043. [DOI] [PubMed] [Google Scholar]
- Schellart N. A., Spekreijse H. Origin of the stochastic nature of ganglion cell activity in isolated goldfish retina. Vision Res. 1973 Feb;13(2):337–345. doi: 10.1016/0042-6989(73)90111-9. [DOI] [PubMed] [Google Scholar]
- Shapley R. M., Victor J. D. How the contrast gain control modifies the frequency responses of cat retinal ganglion cells. J Physiol. 1981 Sep;318:161–179. doi: 10.1113/jphysiol.1981.sp013856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevi Y. Y., Mangoubi S. S. Noise suppression in photoreceptors and its relevance to incremental intensity thresholds. J Opt Soc Am. 1978 Dec;68(12):1772–1776. doi: 10.1364/josa.68.001772. [DOI] [PubMed] [Google Scholar]


